PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures
- PMID: 23060885
- PMCID: PMC3463874
- DOI: 10.3389/fimmu.2012.00307
PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures
Abstract
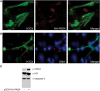
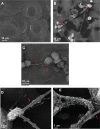
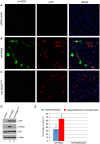
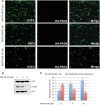
NETosis, the process wherein neutrophils release highly decondensed chromatin called neutrophil extracellular traps (NETs), has gained much attention as an alternative means of killing bacteria. In vivo, NETs are induced by bacteria and pro-inflammatory cytokines. We have reported that peptidylarginine deiminase 4 (PAD4), an enzyme that converts Arg or monomethyl-Arg to citrulline in histones, is essential for NET formation. The areas of extensive chromatin decondensation along the NETs were rich in histone citrullination. Here, upon investigating the effect of global citrullination in cultured cells, we discovered that PAD4 overexpression in osteosarcoma U2OS cells induces extensive chromatin decondensation independent of apoptosis. The highly decondensed chromatin is released to the extracellular space and stained strongly by a histone citrulline-specific antibody. The structure of the decondensed chromatin is reminiscent of NETs but is unique in that it occurs without stimulation of cells with pro-inflammatory cytokines and bacteria. Furthermore, histone citrullination during chromatin decondensation can dissociate heterochromatin protein 1 beta (HP1β) thereby offering a new molecular mechanism for understanding how citrullination regulates chromatin function. Taken together, our study suggests that PAD4 mediated citrullination induces chromatin decondensation, implicating its essential role in NET formation under physiological conditions in neutrophils.
Keywords: chromatin decondensation; heterochromatin protein 1; histone modifications; hypercitrullination; neutrophil extracellular traps; pad4.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

