Polarization of calcium signaling and fluid secretion in salivary gland cells
- PMID: 23061636
- PMCID: PMC3840904
- DOI: 10.2174/092986712804143321
Polarization of calcium signaling and fluid secretion in salivary gland cells
Abstract
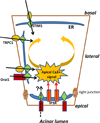
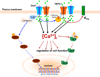
The secretion of fluid, electrolytes, and protein by exocrine gland acinar cells is a vectorial process that requires the coordinated regulation of multiple channel and transporter proteins, signaling components, as well as mechanisms involved in vesicular fusion and water transport. Most critical in this is the regulation of cytosolic free [Ca(2+)] ([Ca(2+)](i)) in response to neurotransmitter stimulation. Control of [Ca(2+)](i) increase in specific regions of the cell is the main determinant of fluid and electrolyte secretion in salivary gland acinar cells as it regulates several major ion flux mechanisms as well as the water channel that are required for this process. Polarized [Ca(2+)](i) signals are also essential for protein secretion in pancreatic acinar cells. Thus, the mechanisms that generate and modulate these compartmentalized [Ca(2+)](i) signals are central to the regulation of exocrine secretion. These mechanisms include membrane receptors for neurotransmitters, intracellular Ca(2+) release channels, Ca(2+) entry channels, as well Ca(2+) as pumps and mitochondria. The spatial arrangement of proteins involved in Ca(2+) signaling is of primary significance in the generation of specific compartmentalized [Ca(2+)](i) signals. Within these domains, both local and global [Ca(2+)](i) changes are tightly controlled. Control of secretion is also dependent on the targeting of ion channels and transporters to specific domains in the cell where their regulation by [Ca(2+)](i) signals is facilitated. Together, the polarized localization of Ca(2+) signaling and secretory components drive vectorial secretion of fluid, electrolytes, and proteins in the exocrine salivary glands and pancreas. This review will discuss recent findings which have led to resolution of the molecular components underlying the spatio-temporal control of [Ca(2+)](i) signals in exocrine gland cells and their role in secretion.
Conflict of interest statement
The author(s) confirm that this article content has no conflicts of interest.
Figures

References
-
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. - PubMed
-
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. - PubMed
-
- Mikoshiba K, Hisatsune C, Futatsugi A, Mizutani A, Nakamura T, Miyachi K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea. 2008;27(Suppl 1):S3–S8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

