Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride
- PMID: 23061667
- PMCID: PMC3494465
- DOI: 10.1021/ja3084797
Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride
Abstract
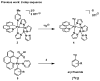
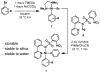
A one-step oxidative fluorination for carbon-fluorine bond formation from well-defined nickel complexes with oxidant and aqueous fluoride is presented, which enables a straightforward and practical (18)F late-stage fluorination of complex small molecules with potential for PET imaging.
Figures
References
-
- Miller PW, Long NJ, Vilar R, Gee AD. Angew Chem Int Ed. 2008;47:8998–9033. - PubMed
-
-
For the first transition metal-catalyzed C–18F bond formation, see Teare H, Robins EG, Kirjavainen A, Forsback S, Sandford G, Solin O, Luthra SK, Gouverneur V. Angew Chem Int Ed. 2010;49:6821–6824.For other successful recent C–18F bond formation, see: Hollingworth C, Hazari A, Hopkinson MN, Tredwell M, Benedetto E, Huiban M, Gee AD, Brown JM, Gouverneur V. Angew Chem Int Ed. 2011;50:2613–2617.Topczewski JJ, Tewson TJ, Nguyen HM. J Am Chem Soc. 2011;133:19318–19321.Gao ZH, Lim YH, Tredwell M, Li L, Verhoog S, Hopkinson M, Kaluza W, Collier TL, Passchier J, Huiban M, Gouverneur V. Angew Chem Int Ed. 2012;51:6733–6737.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources