Understanding and targeting osteoclastic activity in prostate cancer bone metastases
- PMID: 23061677
- PMCID: PMC3624036
- DOI: 10.2174/1566524011313040012
Understanding and targeting osteoclastic activity in prostate cancer bone metastases
Abstract
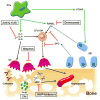
Bone metastasis is a debilitating side effect of advanced prostatic carcinoma impacting nearly all of the men developing this disease. Even though a majority of these lesions are considered osteoblastic, it is believed that there is an underlying osteolytic component. Lytic processes are governed primarily by osteoclasts, the primary bone resorptive cell. Osteolysis has been implicated in tumor cell seeding and nourishment of tumor growth via development of pro-tumorigenic changes in the microenvironment. Herein, we provide a current view of the processes involved in regulating osteolysis in the presence of prostate cancer bone metastases. Several factors have been implicated in the division, differentiation, and activation of osteoclasts, including, but not limited to, interleukin-6, receptor activator of nuclear factor kappa B ligand (RANKL), osteoprotegerin (OPG), and parathyroid hormone-related protein (PTHrP). Effector molecules in bone resorption play a significant role, such as matrix metalloproteinases (MMPs), cathepsins, and acid secretion. The primary method for treating skeletal events associated with prostate cancer bone metastases has been bisphosphonates. However, a new therapeutic, denosumab, a monoclonal antibody that inhibits RANKL in a mechanism similar to that attributed to the endogenous mediator OPG, has received approval for treatment of skeletally associated metastases. Additional novel targets are continuously being developed for bone metastases. In this review, we describe the processes involved in osteolysis of the prostate cancer bone microenvironment, and introduce therapeutics that may play a role in inhibiting tumor growth leading to increased survival and quality of life.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures

References
-
- Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000 Jun 15;88(12 Suppl):2989–94. - PubMed
-
- Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. Journal of cellular biochemistry. 2004 Mar 1;91(4):718–29. - PubMed
-
- Roland SI. Calcium studies in ten cases of osteoblastic prostatic metastasis. The Journal of urology. 1958 Feb;79(2):339–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
