Trends in the use of antiepileptic drugs among pregnant women in the US, 2001-2007: a medication exposure in pregnancy risk evaluation program study
- PMID: 23061694
- PMCID: PMC3481178
- DOI: 10.1111/ppe.12004
Trends in the use of antiepileptic drugs among pregnant women in the US, 2001-2007: a medication exposure in pregnancy risk evaluation program study
Abstract
Background: Little is known about the extent of antiepileptic drug (AED) use in pregnancy, particularly for newer agents. Our objective was to assess whether AED use has increased among pregnant women in the US, 2001-2007.
Methods: We analysed data from the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP) database, 1 January 2001 to 31 December 2007. We identified liveborn deliveries among women, aged 15-45 years on delivery date, who were members of MEPREP health plans (n=585615 deliveries). Pregnancy exposure to AEDs, determined through outpatient pharmacy dispensing files. Older AEDs were available for clinical use before 1993; other agents were considered newer AEDs. Information on sociodemographic and medical/reproductive factors was obtained from linked birth certificate files. Maternal diagnoses were identified based on ICD-9 codes.
Results: Prevalence of AED use during pregnancy increased between 2001 (15.7 per 1000 deliveries) and 2007 (21.9 per 1000 deliveries), driven primarily by a fivefold increase in the use of newer AEDs. Thirteen per cent of AED-exposed deliveries involved a combination of two or more AEDs. Psychiatric disorders were the most prevalent diagnoses, followed by epileptic and pain disorders, among AED users regardless of AED type, year of conception or gestational period.
Conclusions: AED use during pregnancy increased between 2001 and 2007, driven by a fivefold increase in the use of newer AEDs. Nearly one in eight AED-exposed deliveries involved the concomitant use of more than one AED. Additional investigations of the reproductive safety of newer AEDs may be needed.
© 2012 Blackwell Publishing Ltd.
Figures
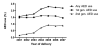
References
-
- Spina E, Perugi G. Antiepileptic drugs: indications other than epilepsy. Epileptic Disorders. 2004;6:57–75. - PubMed
-
- Utilization of antiepileptic drugs during pregnancy: comparative patterns in 38 countries based on data from the EURAP registry. Epilepsia. 2009;50:2305–2309. - PubMed
-
- Vajda FJ, Hollingworth S, Graham J, Hitchcock AA, O’Brien TJ, Lander CM, et al. Changing patterns of antiepileptic drug use in pregnant Australian women. Acta Neurologica Scandinavica. 2010;121:89–93. - PubMed
-
- Cunnington MC, Weil JG, Messenheimer JA, Ferber S, Yerby M, Tennis P. Final results from 18 years of the International Lamotrigine Pregnancy Registry. Neurology. 2011;76:1817–1823. - PubMed
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurology. 2011;10:609–617. - PubMed

