The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice
- PMID: 23062036
- PMCID: PMC3624793
- DOI: 10.2174/1574884711308020006
The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice
Abstract
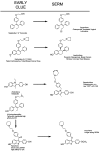
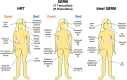
Selective estrogen receptor modulators (SERMs) are structurally different compounds that interact with intracellular estrogen receptors in target organs as estrogen receptor agonists or antagonists. These drugs have been intensively studied over the past decade and have proven to be a highly versatile group for the treatment of different conditions associated with postmenopausal women's health, including hormone responsive cancer and osteoporosis. Tamoxifen, a failed contraceptive is currently used to treat all stages of breast cancer, chemoprevention in women at high risk for breast cancer and also has beneficial effects on bone mineral density and serum lipids in postmenopausal women. Raloxifene, a failed breast cancer drug, is the only SERM approved internationally for the prevention and treatment of postmenopausal osteoporosis and vertebral fractures. However, although these SERMs have many benefits, they also have some potentially serious adverse effects, such as thromboembolic disorders and, in the case of tamoxifen, uterine cancer. These adverse effects represent a major concern given that long-term therapy is required to prevent osteoporosis or prevent and treat breast cancer. The search for the 'ideal' SERM, which would have estrogenic effects on bone and serum lipids, neutral effects on the uterus, and antiestrogenic effects on breast tissue, but none of the adverse effects associated with current therapies, is currently under way. Ospemifene, lasofoxifene, bazedoxifene and arzoxifene, which are new SERM molecules with potentially greater efficacy and potency than previous SERMs, have been investigated for use in the treatment and prevention of osteoporosis. These drugs have been shown to be comparably effective to conventional hormone replacement therapy in animal models, with potential indications for an improved safety profile. Clinical efficacy data from ongoing phase III trials are available or are awaited for each SERM so that a true understanding of the therapeutic potential of these compounds can be obtained. In this article, we describe the discovery and development of the group of medicines called SERMs. The newer SERMs in late development: ospemifene, lasofoxifene, bazedoxifene, are arzoxifene are described in detail.
Figures



References
-
- Lacassagne A. Hormonal pathogenesis of adenocarcinoma of the breast. Am J Cancer. 1936;27:217–25.
-
- Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–89. - PubMed
-
- Lerner LJ, Holthaus FJ, Jr, Thompson CR. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958;63:295–318. - PubMed
-
- Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. JAMA. 1961;178:101–4. - PubMed
-
- Greenblatt RB, Roy S, Mahesh VB. Induction of ovulation. Am J Obstet Gynecol. 1962;84:900–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

