A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia
- PMID: 23062189
- PMCID: PMC3580529
- DOI: 10.1186/ar4056
A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia
Abstract
Introduction: Fibromyalgia is a chronic disorder characterized by widespread pain and tenderness. Prior trials have demonstrated the efficacy of pregabalin for the relief of fibromyalgia symptoms, and it is approved for the treatment of fibromyalgia in the United States. However, prior to this study, there has not been a large-scale efficacy trial in patients with fibromyalgia in Japan.
Methods: This randomized, double-blind, multicenter, placebo-controlled trial was conducted at 44 centers in Japan to assess the efficacy and safety of pregabalin for the symptomatic relief of pain in fibromyalgia patients. Patients aged ≥18 years who had met the criteria for fibromyalgia were randomized to receive either pregabalin, starting at 150 mg/day and increasing to a maintenance dose of 300 or 450 mg/day, or placebo, for 15 weeks. The primary efficacy endpoint was mean pain score at final assessment. Secondary endpoints included Patient Global Impression of Change (PGIC) together with measures of sleep, physical functioning and quality of life.
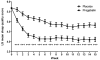
Results: A total of 498 patients (89% female) were randomized to receive either pregabalin (n = 250) or placebo (n = 248). Pregabalin significantly reduced mean pain score at final assessment (difference in mean change from baseline, compared with placebo -0.44; P = 0.0046) and at every week during the study (P <0.025). Key secondary endpoints were also significantly improved with pregabalin treatment compared with placebo, including PGIC (percentage reporting symptoms "very much improved" or "much improved", 38.6% vs 26.7% with placebo; P = 0.0078); pain visual analog scale (difference in mean change from baseline, compared with placebo -6.19; P = 0.0013); Fibromyalgia Impact Questionnaire total score (-3.33; P = 0.0144); and quality of sleep score (-0.73; P <0.0001). Treatment was generally well tolerated, with somnolence and dizziness the most frequently reported adverse events.
Conclusions: This trial demonstrated that pregabalin, at doses of up to 450 mg/day, was effective for the symptomatic relief of pain in Japanese patients with fibromyalgia. Pregabalin also improved measures of sleep and functioning and was well tolerated. These data indicate that pregabalin is an effective treatment option for the relief of pain and sleep problems in Japanese patients with fibromyalgia.
Trial registration: ClinicalTrials.gov: NCT00830167.
Figures




References
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. et al.The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. - DOI - PubMed
-
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl. 2005;75:6–21. - PubMed
-
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

