Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cells via stimulation of interleukin-8 autocrine secretion
- PMID: 23062209
- PMCID: PMC4053109
- DOI: 10.1186/bcr3329
Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cells via stimulation of interleukin-8 autocrine secretion
Abstract
Introduction: The tyrosine kinase receptors HER2 and HER3 play an important role in breast cancer. The HER2/HER3 heterodimer is a critical oncogenic unit associated with reduced relapse-free and decreased overall survival. While signaling cascades downstream of HER2 and HER3 have been studied extensively at the level of post-translational modification, little is known about the effects of HER2/HER3 overexpression and activation on gene expression in breast cancer. We have now defined the genetic landscape induced by activation of the HER2/HER3 unit in mammary cells, and have identified interleukin (IL)8 and CXCR1 as potential therapeutic targets for the treatment of HER2/HER3-overexpressing breast cancers.
Methods: Three-dimensional (3D) cultures, invasion and migration assays were used to determine the effects of HER2 and HER3 co-expression and activation. Gene expression analysis was performed to identify the gene network induced by HER2/HER3 in 3D cultures. Bioinformatic analysis and neutralizing antibodies were used to identify key mediators of HER2/HER3-evoked invasion.
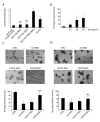
Results: Co-expression of the tyrosine kinase receptors HER2 and HER3 induced migration and invasion of MCF10A cells. Microarray analysis of these cells revealed a specific "HER2/HER3 signature" comprising 80 upregulated transcripts, with IL8 being the highest (11-fold upregulation). Notably, examination of public datasets revealed high levels of IL8 transcripts in HER2-enriched as well as basal-like primary breast tumors, two subtypes characterized by a particularly poor prognosis. Moreover, IL8 expression correlated with high tumor grade and ER-negative status. Importantly, treatment with IL8-neutralizing antibodies prevented invasion of MCF10A-HER2/HER3 and BT474 cells in 3D cultures, highlighting the importance of IL8 autocrine signaling upon HER2/HER3 activation.
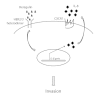
Conclusions: Our findings demonstrate that HER2 and HER3 co-expression induces IL8 autocrine signaling, leading to the invasion of mammary cells. Agents targeting IL8 or its receptor CXCR1 may be useful for the treatment of HER2/HER3/IL8-positive breast cancers with invasive traits.
Figures



References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;14:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

