A modular fibrinogen model that captures the stress-strain behavior of fibrin fibers
- PMID: 23062346
- PMCID: PMC3471473
- DOI: 10.1016/j.bpj.2012.08.038
A modular fibrinogen model that captures the stress-strain behavior of fibrin fibers
Abstract
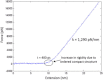
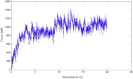
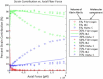
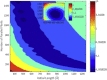
We tested what to our knowledge is a new computational model for fibrin fiber mechanical behavior. The model is composed of three distinct elements: the folded fibrinogen core as seen in the crystal structure, the unstructured α-C connector, and the partially folded α-C domain. Previous studies have highlighted the importance of all three regions and how they may contribute to fibrin fiber stress-strain behavior. Yet no molecular model has been computationally tested that takes into account the individual contributions of all these regions. Constant velocity, steered molecular dynamics studies at 0.025 Å/ps were conducted on the folded fibrinogen core and the α-C domain to determine their force-displacement behavior. A wormlike chain model with a persistence length of 0.8 nm (Kuhn length = 1.6 nm) was used to model the mechanical behavior of the unfolded α-C connector. The three components were combined to calculate the total stress-strain response, which was then compared to experimental data. The results show that the three-component model successfully captures the experimentally determined stress-strain behavior of fibrin fibers. The model evinces the key contribution of the α-C domains to fibrin fiber stress-strain behavior. However, conversion of the α-helical coiled coils to β-strands, and partial unfolding of the protein, may also contribute.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Recombinant fibrinogen reveals the differential roles of α- and γ-chain cross-linking and molecular heterogeneity in fibrin clot strain-stiffening.J Thromb Haemost. 2017 May;15(5):938-949. doi: 10.1111/jth.13650. Epub 2017 Mar 6. J Thromb Haemost. 2017. PMID: 28166607
-
Molecular basis of fibrin clot elasticity.Structure. 2008 Mar;16(3):449-59. doi: 10.1016/j.str.2007.12.019. Epub 2008 Feb 21. Structure. 2008. PMID: 18294856
-
Variability in individual native fibrin fiber mechanics.Phys Biol. 2024 Oct 30;21(6). doi: 10.1088/1478-3975/ad899f. Phys Biol. 2024. PMID: 39433274
-
A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers.Cell Biochem Biophys. 2007;49(3):165-81. doi: 10.1007/s12013-007-9001-4. Epub 2007 Oct 2. Cell Biochem Biophys. 2007. PMID: 17952642 Free PMC article. Review.
-
The molecular origins of the mechanical properties of fibrin.Biophys Chem. 2010 Nov;152(1-3):15-20. doi: 10.1016/j.bpc.2010.08.009. Biophys Chem. 2010. PMID: 20888119 Free PMC article. Review.
Cited by
-
Rupture mechanics of blood clot fibrin fibers: A coarse-grained model study.J Mech Phys Solids. 2025 Mar;196:105998. doi: 10.1016/j.jmps.2024.105998. Epub 2024 Dec 2. J Mech Phys Solids. 2025. PMID: 39734807 Free PMC article.
-
Elastic behavior and platelet retraction in low- and high-density fibrin gels.Biophys J. 2015 Jan 6;108(1):173-83. doi: 10.1016/j.bpj.2014.11.007. Biophys J. 2015. PMID: 25564864 Free PMC article.
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Multiscale Network Modeling of Fibrin Fibers and Fibrin Clots with Protofibril Binding Mechanics.Polymers (Basel). 2020 May 27;12(6):1223. doi: 10.3390/polym12061223. Polymers (Basel). 2020. PMID: 32471225 Free PMC article.
-
Finite element analysis of blood clots based on the nonlinear visco-hyperelastic model.Biophys J. 2021 Oct 19;120(20):4547-4556. doi: 10.1016/j.bpj.2021.08.034. Epub 2021 Sep 1. Biophys J. 2021. PMID: 34478700 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

