A biophysical model of the mitochondrial ATP-Mg/P(i) carrier
- PMID: 23062354
- PMCID: PMC3471468
- DOI: 10.1016/j.bpj.2012.08.050
A biophysical model of the mitochondrial ATP-Mg/P(i) carrier
Abstract
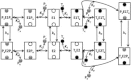
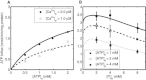
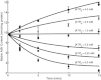
Mitochondrial adenine nucleotide (AdN) content is regulated through the Ca(2+)-activated, electroneutral ATP-Mg/P(i) carrier (APC). The APC is a protein in the mitochondrial carrier super family that localizes to the inner mitochondrial membrane (IMM). It is known to modulate a number of processes that depend on mitochondrial AdN content, such as gluconeogenesis, protein synthesis, and citrulline synthesis. Despite this critical role, a kinetic model of the underlying mechanism has not been developed and validated. Here, a biophysical model of the APC is developed that is thermodynamically balanced and accurately reproduces a number of reported data sets from isolated rat liver and rat kidney mitochondria. The model is based on an ordered bi-bi mechanism for heteroexchange of ATP and P(i) and includes homoexchanges of ATP and P(i) to explain both the initial rate and time course data on ATP and P(i) transport via the APC. The model invokes seven kinetic parameters regarding the APC mechanism and three parameters related to matrix pH regulation by external P(i). These parameters are estimated based on 19 independent data curves; the estimated parameters are validated using six additional data curves. The model takes into account the effects of pH, Mg(2+), and Ca(2+) on ATP and P(i) transport via the APC, and supports the conclusion that the pH gradient across the IMM serves as the primary driving force for AdN uptake or efflux. Moreover, computer simulations demonstrate that extramatrix Ca(2+) modulates the turnover rate of the APC and not the binding affinity of ATP, as previously suggested.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Net adenine nucleotide transport in rat kidney mitochondria.Arch Biochem Biophys. 1993 Jun;303(2):195-207. doi: 10.1006/abbi.1993.1273. Arch Biochem Biophys. 1993. PMID: 8512308
-
ATP-Mg/Pi carrier activity in rat liver mitochondria.Arch Biochem Biophys. 1992 Aug 1;296(2):691-7. doi: 10.1016/0003-9861(92)90628-a. Arch Biochem Biophys. 1992. PMID: 1632654
-
Reversible and irreversible mitochondrial swelling in vitro.Biophys Chem. 2021 Nov;278:106668. doi: 10.1016/j.bpc.2021.106668. Epub 2021 Aug 14. Biophys Chem. 2021. PMID: 34418677
-
Regulation of the mitochondrial adenine nucleotide pool size in liver: mechanism and metabolic role.FASEB J. 1988 Jul;2(10):2547-56. doi: 10.1096/fasebj.2.10.3290024. FASEB J. 1988. PMID: 3290024 Review.
-
Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
Cited by
-
SLC25A23 augments mitochondrial Ca²⁺ uptake, interacts with MCU, and induces oxidative stress-mediated cell death.Mol Biol Cell. 2014 Mar;25(6):936-47. doi: 10.1091/mbc.E13-08-0502. Epub 2014 Jan 15. Mol Biol Cell. 2014. PMID: 24430870 Free PMC article.
-
Determination of the catalytic mechanism for mitochondrial malate dehydrogenase.Biophys J. 2015 Jan 20;108(2):408-19. doi: 10.1016/j.bpj.2014.11.3467. Biophys J. 2015. PMID: 25606688 Free PMC article.
-
Molecular regulation of MCU: Implications in physiology and disease.Cell Calcium. 2018 Sep;74:86-93. doi: 10.1016/j.ceca.2018.06.006. Epub 2018 Jun 27. Cell Calcium. 2018. PMID: 29980025 Free PMC article. Review.
-
A self-sequestered calmodulin-like Ca²⁺ sensor of mitochondrial SCaMC carrier and its implication to Ca²⁺-dependent ATP-Mg/P(i) transport.Structure. 2014 Feb 4;22(2):209-17. doi: 10.1016/j.str.2013.10.018. Epub 2013 Dec 12. Structure. 2014. PMID: 24332718 Free PMC article.
-
Agent-based modeling of neuronal mitochondrial dynamics using intrinsic variables of individual mitochondria.iScience. 2025 Apr 8;28(5):112390. doi: 10.1016/j.isci.2025.112390. eCollection 2025 May 16. iScience. 2025. PMID: 40330889 Free PMC article.
References
-
- Aprille J.R. Mechanism and regulation of the mitochondrial ATP-Mg/Pi carrier. J. Bioenerg. Biomembr. 1993;25:473–481. - PubMed
-
- Satrústegui J., Pardo B., Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol. Rev. 2007;87:29–67. - PubMed
-
- Hagen T., Joyal J.L., Aprille J.R. Net adenine nucleotide transport in rat kidney mitochondria. Arch. Biochem. Biophys. 1993;303:195–207. - PubMed
-
- Fiermonte G., De Leonardis F., Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 2004;279:30722–30730. - PubMed
-
- Joyal J.L., Aprille J.R. The ATP-Mg/Pi carrier of rat liver mitochondria catalyzes a divalent electroneutral exchange. J. Biol. Chem. 1992;267:19198–19203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

