System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197
- PMID: 23063032
- PMCID: PMC3570840
- DOI: 10.1177/193229681200600510
System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197
Abstract
Background: The accuracy of systems for self-monitoring of blood glucose is important, as reliable measurement results are a prerequisite for therapeutic decisions.
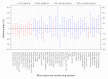
Methods: This system accuracy evaluation study was performed according to DIN EN ISO 15197:2003 for 43 Conformité Européenne (CE)-labeled blood glucose (BG) monitoring systems. Measurement results of each system were compared with results of the designated comparison method (manufacturer's measurement procedure): glucose oxidase method (YSI 2300 glucose analyzer) or hexokinase method (Hitachi 917/ cobas 501).
Results: Complete assessment according to the International Organization for Standardization (ISO) standard was performed for 34 out of 43 systems, and 27 (79.4%) meet the requirements of the standard, i.e., ≥95% of their results showed at least the minimum acceptable accuracy. For 9 of the 43 systems, complete accuracy assessment was not performed due to an oxygen sensitivity (manufacturer's labeling). The bias (according to Bland and Altman) of all 43 evaluated systems ranged from -14.1% to +12.4%.
Conclusions: From the 34 systems completely assessed, 7 systems did not fulfill the minimal accuracy requirements of the ISO standard. The CE mark apparently does not guarantee that all BG systems provide accuracy according to the standard. Because inaccurate systems bear the risk of false therapeutic decisions, regular and standardized evaluation of BG meters and test strips should be requested in order to ensure adherence to quality standards.
© 2012 Diabetes Technology Society.
Figures



Comment in
-
System accuracy of blood glucose monitoring devices according to the current and proposed ISO 15197 standards.J Diabetes Sci Technol. 2013 May 1;7(3):795-7. doi: 10.1177/193229681300700325. J Diabetes Sci Technol. 2013. PMID: 23759413 Free PMC article. No abstract available.
References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus N Engl J Med. 1993;329(14):977–986. - PubMed
-
- Blonde L, Karter AJ. Current evidence regarding the value of self-monitored blood glucose testing. Am J Med. 2005;118((Suppl 9A)):20S–26S. - PubMed
-
- IDF Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23(6):579–593. - PubMed
-
- Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. - PubMed
-
- Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatr Diabetes. 2006;7(3):159–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

