GlucoMen Day continuous glucose monitoring system: a screening for enzymatic and electrochemical interferents
- PMID: 23063044
- PMCID: PMC3570852
- DOI: 10.1177/193229681200600522
GlucoMen Day continuous glucose monitoring system: a screening for enzymatic and electrochemical interferents
Abstract
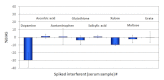
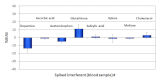
Background: While most of the common drugs with the potential to interfere with continuous glucose monitoring (CGM) systems are accessible over the counter and can be assumed by CGM patients without medical supervision, many other chemicals are frequently used to treat critically ill patients. Continuous glucose monitoring reading accuracy may also be compromised in patients characterized by abnormally high concentrations of physiological interferents. In this article, 22 species selected from endogenous and exogenous chemicals were screened as possible interferents of GlucoMen®Day (GMD), the new microdialysis-based CGM system from A. Menarini Diagnostics.
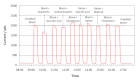
Method: Interference testing was performed according to the EP7-A2 guideline (Clinical and Laboratory Standards Institute 2005). Interference was evaluated at two levels of glucose, with each interferent additionally tested at two concentrations. Furthermore, two configurations of the GMD disposable sensor kit--one designed for subcutaneous application, the other for direct intravascular CGM--were challenged with interferent-spiked serum and blood samples, respectively.
Results: With the exception of dopamine (however, at very high, nonphysiological concentrations), no interference was observed for all the tested substances. Interestingly, none of the common electrochemical interferents (including ascorbic acid, acetaminophen, and salicylic acid, which represent the major specificity issue for the competing CGM systems) significantly affected the system's output.
Conclusions: These results provide clear insights into the advantages offered by the use of a microdialysis-based CGM system that additionally relies on the detection of hydrogen peroxide at low operating potential. GlucoMen Day may become the CGM system of choice for those patients who require either regular administration of drugs or their glycemia to be tightly controlled in the intensive care unit or similar environments.
© 2012 Diabetes Technology Society.
Figures

Comment in
-
Interference testing: why following standards is not always the right thing to do.J Diabetes Sci Technol. 2012 Sep 1;6(5):1182-4. doi: 10.1177/193229681200600523. J Diabetes Sci Technol. 2012. PMID: 23063045 Free PMC article.
References
-
- Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108(2):814–825. - PubMed
-
- McGarraugh G. The chemistry of commercial continuous glucose monitors. Diabetes Technol Ther. 2009;11(Suppl 1):S17–24. - PubMed
-
- Feldman B, Brazg R, Schwartz S, Weinstein R. A continuous glucose sensor based on wired enzyme technology — results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther. 2003;5(5):769–779. - PubMed
-
- Cunningham DD, Stenken JA. In vivo glucose sensing. Hoboken: John Wiley & Sons; 2010.
-
- De Block C, Manuel-y-Keenoy B, Van Gaal L, Rogiers P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006;29(8):1750–1756. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

