Interference testing: why following standards is not always the right thing to do
- PMID: 23063045
- PMCID: PMC3570853
- DOI: 10.1177/193229681200600523
Interference testing: why following standards is not always the right thing to do
Abstract
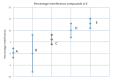
Lucarelli and colleagues in this issue of Journal of Diabetes Science and Technology describe the results of interference testing for a continuous glucose monitoring system. The authors follow the Clinical Laboratory Standards Institute guideline EP7-A2, including their conclusions, in which the concepts of a statistically significant interfering substance and a clinically important interference have been combined in a way whereby information from the experiment has been lost and could be misleading. A better way to treat the data is presented, including a simulation method to evaluate the effects of interferences.
© 2012 Diabetes Technology Society.
Figures
Comment on
-
GlucoMen Day continuous glucose monitoring system: a screening for enzymatic and electrochemical interferents.J Diabetes Sci Technol. 2012 Sep 1;6(5):1172-81. doi: 10.1177/193229681200600522. J Diabetes Sci Technol. 2012. PMID: 23063044 Free PMC article.
References
-
- Clinical and Laboratory Standards Institute. Interference testing in clinical chemistry; approved guideline—second edition. CLSI document EP7-A2. Wayne: Clinical and Laboratory Standards Institute; 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

