Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition
- PMID: 23063123
- PMCID: PMC4175720
- DOI: 10.1016/j.cell.2012.09.004
Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition
Abstract
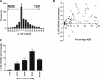
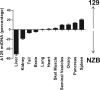
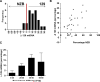
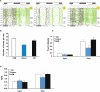
Maternal inheritance of mtDNA is the rule in most animals, but the reasons for this pattern remain unclear. To investigate the consequence of overriding uniparental inheritance, we generated mice containing an admixture (heteroplasmy) of NZB and 129S6 mtDNAs in the presence of a congenic C57BL/6J nuclear background. Analysis of the segregation of the two mtDNAs across subsequent maternal generations revealed that proportion of NZB mtDNA was preferentially reduced. Ultimately, this segregation process produced NZB-129 heteroplasmic mice and their NZB or 129 mtDNA homoplasmic counterparts. Phenotypic comparison of these three mtDNA lines demonstrated that the NZB-129 heteroplasmic mice, but neither homoplasmic counterpart, had reduced activity, food intake, respiratory exchange ratio; accentuated stress response; and cognitive impairment. Therefore, admixture of two normal but different mouse mtDNAs can be genetically unstable and can produce adverse physiological effects, factors that may explain the advantage of uniparental inheritance of mtDNA.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

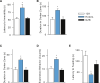
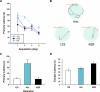
Comment in
-
The problem with mixing mitochondria.Cell. 2012 Oct 12;151(2):246-8. doi: 10.1016/j.cell.2012.09.028. Cell. 2012. PMID: 23063117
-
Chromosome biology: mixing it up.Nat Rev Mol Cell Biol. 2012 Dec;13(12):750. doi: 10.1038/nrm3476. Epub 2012 Nov 15. Nat Rev Mol Cell Biol. 2012. PMID: 23151661 No abstract available.
References
-
- Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–1147. - PubMed
-
- Alonso SJ, Castellano MA, Afonso D, Rodriguez M. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol. Behav. 1991;49:69–72. - PubMed
-
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. - PubMed
-
- Battersby BJ, Shoubridge EA. Selection of a mtDNA sequence variant in hepatocytes of heteroplasmic mice is not due to differences in respiratory chain function or efficiency of replication. Hum. Mol. Genet. 2001;10:2469–2479. - PubMed
-
- Battersby BJ, Loredo-Osti JC, Shoubridge EA. Nuclear genetic control of mitochondrial DNA segregation. Nat. Genet. 2003;33:183–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

