The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation
- PMID: 23063526
- PMCID: PMC3513569
- DOI: 10.1016/j.molcel.2012.08.033
The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation
Abstract
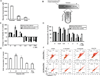
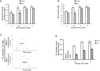
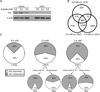
Widespread changes in gene expression drive tumorigenesis, yet our knowledge of how aberrant epigenomic and transcriptome profiles arise in cancer cells is poorly understood. Here, we demonstrate that metabolic transformation plays an important role. Butyrate is the primary energy source of normal colonocytes and is metabolized to acetyl-CoA, which was shown to be important not only for energetics but also for HAT activity. Due to the Warburg effect, cancerous colonocytes rely on glucose as their primary energy source, so butyrate accumulated and functioned as an HDAC inhibitor. Although both mechanisms increased histone acetylation, different target genes were upregulated. Consequently, butyrate stimulated the proliferation of normal colonocytes and cancerous colonocytes when the Warburg effect was prevented from occurring, whereas it inhibited the proliferation of cancerous colonocytes undergoing the Warburg effect. These findings link a common metabolite to epigenetic mechanisms that are differentially utilized by normal and cancerous cells because of their inherent metabolic differences.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Comment in
-
Metabolism: Warburg behind the butyrate paradox?Nat Rev Cancer. 2012 Dec;12(12):798. doi: 10.1038/nrc3401. Epub 2012 Nov 15. Nat Rev Cancer. 2012. PMID: 23151604 No abstract available.
References
-
- Andriamihaja M, Chaumontet C, Tome D, Blachier F. Butyrate metabolism in human colon carcinoma cells: implications concerning its growth-inhibitory effect. Journal of cellular physiology. 2009;218:58–65. - PubMed
-
- Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. Journal of gastroenterology and hepatology. 2008;23:1298–1303. - PubMed
-
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. The Journal of biological chemistry. 2002;277:33895–33900. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

