Gravin is a transitory effector of polo-like kinase 1 during cell division
- PMID: 23063527
- PMCID: PMC3513578
- DOI: 10.1016/j.molcel.2012.09.002
Gravin is a transitory effector of polo-like kinase 1 during cell division
Abstract
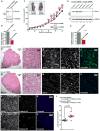
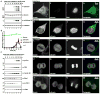
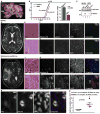
The mitogenic and second-messenger signals that promote cell proliferation often proceed through multienzyme complexes. The kinase-anchoring protein Gravin integrates cAMP and calcium/phospholipid signals at the plasma membrane by sequestering protein kinases A and C with G protein-coupled receptors. In this report we define a role for Gravin as a temporal organizer of phosphorylation-dependent protein-protein interactions during mitosis. Mass spectrometry, molecular, and cellular approaches show that CDK1/Cyclin B1 phosphorylates Gravin on threonine 766 to prime the recruitment of the polo-like kinase Plk1 at defined phases of mitosis. Fluorescent live-cell imaging reveals that cells depleted of Gravin exhibit mitotic defects that include protracted prometaphase and misalignment of chromosomes. Moreover, a Gravin T766A phosphosite mutant that is unable to interact with Plk1 negatively impacts cell proliferation. In situ detection of phospho-T766 Gravin in biopsy sections of human glioblastomas suggests that this phosphorylation event might identify malignant neoplasms.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Ahn NG. PORE-ing over ERK substrates. Nat Struct Mol Biol. 2009;16:1004–1005. - PubMed
-
- Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, et al. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal. 2011;4:ra42. http://dx.doi.org/10.1126/scisignal.2001796. - DOI - PMC - PubMed
-
- Brennan IM, Peters U, Kapoor TM, Straight AF. Polo-like kinase controls vertebrate spindle elongation and cytokinesis. PLoS ONE. 2007;2:e409. http://dx.doi.org/10.1371/journal.pone.0000409. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

