eIF2γ mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation
- PMID: 23063529
- PMCID: PMC3513554
- DOI: 10.1016/j.molcel.2012.09.005
eIF2γ mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation
Abstract
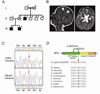
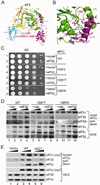
Together with GTP and initiator methionyl-tRNA, translation initiation factor eIF2 forms a ternary complex that binds the 40S ribosome and then scans an mRNA to select the AUG start codon for protein synthesis. Here, we show that a human X-chromosomal neurological disorder characterized by intellectual disability and microcephaly is caused by a missense mutation in eIF2γ (encoded by EIF2S3), the core subunit of the heterotrimeric eIF2 complex. Biochemical studies of human cells overexpressing the eIF2γ mutant and of yeast eIF2γ with the analogous mutation revealed a defect in binding the eIF2β subunit to eIF2γ. Consistent with this loss of eIF2 integrity, the yeast eIF2γ mutation impaired translation start codon selection and eIF2 function in vivo in a manner that was suppressed by overexpressing eIF2β. These findings directly link intellectual disability to impaired translation initiation, and provide a mechanistic basis for the human disease due to partial loss of eIF2 function.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS. Leukoencephalopathy with vanishing white matter: a review. J Neuropathol Exp Neurol. 2010;69:987–996. - PubMed
-
- Dmitriev SE, Stolboushkina EA, Terenin IM, Andreev DE, Garber MB, Shatsky IN. Archaeal translation initiation factor aIF2 can substitute for eukaryotic eIF2 in ribosomal scanning during mammalian 48S complex formation. J Mol Biol. 2011;413:106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

