Tandem PHD fingers of MORF/MOZ acetyltransferases display selectivity for acetylated histone H3 and are required for the association with chromatin
- PMID: 23063713
- PMCID: PMC3502708
- DOI: 10.1016/j.jmb.2012.10.004
Tandem PHD fingers of MORF/MOZ acetyltransferases display selectivity for acetylated histone H3 and are required for the association with chromatin
Abstract
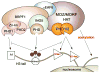
MORF [MOZ (monocytic leukemia zinc-finger protein)-related factor] and MOZ are catalytic subunits of histone acetyltransferase (HAT) complexes essential in hematopoiesis, neurogenesis, skeletogenesis and other developmental programs and implicated in human leukemias. The canonical HAT domain of MORF/MOZ is preceded by a tandem of plant homeodomain (PHD) fingers whose biological roles and requirements for MORF/MOZ activity are unknown. Here, we demonstrate that the tandem PHD1/2 fingers of MORF recognize the N-terminal tail of histone H3. Acetylation of Lys9 (H3K9ac) or Lys14 (H3K14ac) enhances binding of MORF PHD1/2 to unmodified H3 peptides twofold to threefold. The selectivity for acetylated H3 tail is conserved in the double PHD1/2 fingers of MOZ. This interaction requires the intact N-terminus of histone H3 and is inhibited by trimethylation of Lys4. Biochemical analysis using NMR, fluorescence spectroscopy and mutagenesis identified key amino acids of MORF PHD1/2 necessary for the interaction with histones. Fluorescence microscopy and immunoprecipitation experiments reveal that both PHD fingers are required for binding to H3K14ac in vivo and localization to chromatin. The HAT assays indicate that the interaction with H3K14ac may promote enzymatic activity in trans. Together, our data suggest that the PHD1/2 fingers play a role in MOZ/MORF HATs association with the chromatic regions enriched in acetylated marks.
Copyright © 2012. Published by Elsevier Ltd.
Figures




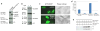
References
-
- Yang XJ, Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene. 2007;26:5408–19. - PubMed
-
- Avvakumov N, Cote J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26:5395–407. - PubMed
-
- Borrow J, Stanton VP, Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dube I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;16)(p11; p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

