A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin
- PMID: 23064014
- PMCID: PMC7958714
- DOI: 10.1210/en.2012-1595
A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin
Abstract
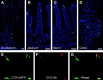
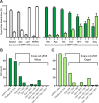
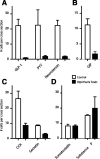
Enteroendocrine cells such as duodenal cholecystokinin (CCK cells) are generally thought to be confined to certain segments of the gastrointestinal (GI) tract and to store and release peptides derived from only a single peptide precursor. In the current study, however, transgenic mice expressing enhanced green fluorescent protein (eGFP) under the control of the CCK promoter demonstrated a distribution pattern of CCK-eGFP positive cells that extended throughout the intestine. Quantitative PCR and liquid chromatography-mass spectrometry proteomic analyses of isolated, FACS-purified CCK-eGFP-positive cells demonstrated expression of not only CCK but also glucagon-like peptide 1 (GLP-1), gastric inhibitory peptide (GIP), peptide YY (PYY), neurotensin, and secretin, but not somatostatin. Immunohistochemistry confirmed this expression pattern. The broad coexpression phenomenon was observed both in crypts and villi as demonstrated by immunohistochemistry and FACS analysis of separated cell populations. Single-cell quantitative PCR indicated that approximately half of the duodenal CCK-eGFP cells express one peptide precursor in addition to CCK, whereas an additional smaller fraction expresses two peptide precursors in addition to CCK. The coexpression pattern was further confirmed through a cell ablation study based on expression of the human diphtheria toxin receptor under the control of the proglucagon promoter, in which activation of the receptor resulted in a marked reduction not only in GLP-1 cells, but also PYY, neurotensin, GIP, CCK, and secretin cells, whereas somatostatin cells were spared. Key elements of the coexpression pattern were confirmed by immunohistochemical double staining in human small intestine. It is concluded that a lineage of mature enteroendocrine cells have the ability to coexpress members of a group of functionally related peptides: CCK, secretin, GIP, GLP-1, PYY, and neurotensin, suggesting a potential therapeutic target for the treatment and prevention of diabetes and obesity.
Figures




References
-
- Schonhoff SE, Giel-Moloney M, Leiter AB 2004. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 145:2639–2644 - PubMed
-
- Rehfeld JF 1998. The new biology of gastrointestinal hormones. Physiol Rev 78:1087–1108 - PubMed
-
- Miller LJ 2003. Gastrointestinal hormones and receptors. In: , Yamada T, ed. Textbook of gastroenterology. Baltimore: Lippincott Williams & Wilkins; 48–76
-
- Field BC, Chaudhri OB, Bloom SR 2010. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol 6:444–453 - PubMed
-
- Sundler F 2004. GI tract, general anatomy (cells). In: , Martini L, ed. Encyclopedia of endocrine diseases. New York: Elsevier; 208. –215
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

