Neurodevelopmental impairment following neonatal hyperoxia in the mouse
- PMID: 23064437
- PMCID: PMC3534920
- DOI: 10.1016/j.nbd.2012.10.005
Neurodevelopmental impairment following neonatal hyperoxia in the mouse
Abstract
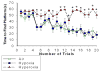
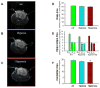
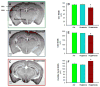
Extremely premature infants are often exposed to supra-physiologic concentrations of oxygen, and frequently have hypoxemic episodes. These preterm infants are at high risk (~40%) for neurodevelopmental impairment (NDI) even in the absence of obvious intracranial pathology such as intraventricular hemorrhage or periventricular leukomalacia. The etiology for NDI has not been determined, and there are no animal models to simulate neurodevelopmental outcomes of prematurity. Our objectives were to develop and characterize a mouse model to determine long-term effects of chronic hypoxia or hyperoxia exposure on neurodevelopment. Newborn C57BL/6 mice were exposed to hypoxia (12% O(2)) or hyperoxia (85% O(2)) from postnatal days 1 to 14 and then returned to air. At 12-14 weeks of age, neurobehavioral assessment (Water Maze test, Novel Object Recognition test, Open Field test, Elevated Plus Maze, and Rotarod test) was performed, followed by MRI and brain histology. Neurobehavioral testing revealed that hyperoxia-exposed mice did poorly on the water maze and novel object recognition tests compared to air-exposed mice. MRI demonstrated smaller hippocampi in hyperoxia- and hypoxia-exposed mice with a greater reduction in hyperoxia-exposed mice, including a smaller cerebellum in hyperoxia-exposed mice. Brain histology showed reduced CA1 and CA3 and increased dentate gyral width in hippocampus. In conclusion, neonatal hyperoxia in mice leads to abnormal neurobehavior, primarily deficits in spatial and recognition memory, associated with smaller hippocampal sizes, similar to findings in ex-preterm infants. This animal model may be useful to determine mechanisms underlying developmental programming of NDI in preterm infants, and for evaluation of therapeutic strategies.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

