The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens
- PMID: 23064463
- PMCID: PMC3493750
- DOI: 10.1038/nm.2920
The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens
Abstract
Allergic asthma is a complex disease characterized by eosinophilic pulmonary inflammation, mucus production and reversible airway obstruction. Exposure to indoor allergens is a risk factor for asthma, but this disease is also associated with high household levels of total and particularly Gram-negative bacteria. The ability of bacterial products to act as adjuvants suggests they might promote asthma by priming allergic sensitization to inhaled allergens. In support of this idea, house dust extracts (HDEs) can activate antigen-presenting dendritic cells (DCs) in vitro and promote allergic sensitization to inhaled innocuous proteins in vivo. It is unknown which microbial products provide most of the adjuvant activity in HDEs. A screen for adjuvant activity of microbial products revealed that the bacterial protein flagellin (FLA) stimulated strong allergic airway responses to an innocuous inhaled protein, ovalbumin (OVA). Moreover, Toll-like receptor 5 (TLR5), the mammalian receptor for FLA, was required for priming strong allergic responses to natural indoor allergens present in HDEs. In addition, individuals with asthma have higher serum levels of FLA-specific antibodies as compared to nonasthmatic individuals. Together, these findings suggest that household FLA promotes the development of allergic asthma by TLR5-dependent priming of allergic responses to indoor allergens.
Figures

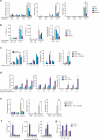
 , increasing amounts (25 ng, 125 ng or 625 ng) of microbial product used during sensitizations; sFLA also includes 1250 ng. n = 5 – 8 mice per group. (d) Pulmonary cytokines: left to right, IL-4, IL-5, IL-13 and IL-17. (e) Total serum IgE. Data are from one of three similar experiments. n = 5 – 8 mice per group. nd, not detected.
, increasing amounts (25 ng, 125 ng or 625 ng) of microbial product used during sensitizations; sFLA also includes 1250 ng. n = 5 – 8 mice per group. (d) Pulmonary cytokines: left to right, IL-4, IL-5, IL-13 and IL-17. (e) Total serum IgE. Data are from one of three similar experiments. n = 5 – 8 mice per group. nd, not detected.

References
-
- Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001;344:350–362. - PubMed
-
- Ross MA, et al. Association of asthma symptoms and severity with indoor bioaerosols. Allergy. 2000;55:705–711. - PubMed
-
- Ng N, Lam D, Paulus P, Batzer G, Horner AA. House dust extracts have both TH2 adjuvant and tolerogenic activities. J Allergy Clin Immunol. 2006;117:1074–1081. - PubMed
-
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. - PubMed

