Transcriptional burst frequency and burst size are equally modulated across the human genome
- PMID: 23064634
- PMCID: PMC3491463
- DOI: 10.1073/pnas.1213530109
Transcriptional burst frequency and burst size are equally modulated across the human genome
Abstract
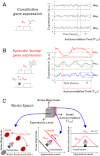
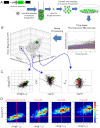
Gene expression occurs either as an episodic process, characterized by pulsatile bursts, or as a constitutive process, characterized by a Poisson-like accumulation of gene products. It is not clear which mode of gene expression (constitutive versus bursty) predominates across a genome or how transcriptional dynamics are influenced by genomic position and promoter sequence. Here, we use time-lapse fluorescence microscopy to analyze 8,000 individual human genomic loci and find that at virtually all loci, episodic bursting--as opposed to constitutive expression--is the predominant mode of expression. Quantitative analysis of the expression dynamics at these 8,000 loci indicates that both the frequency and size of the transcriptional bursts varies equally across the human genome, independent of promoter sequence. Strikingly, weaker expression loci modulate burst frequency to increase activity, whereas stronger expression loci modulate burst size to increase activity. Transcriptional activators such as trichostatin A (TSA) and tumor necrosis factor α (TNF) only modulate burst size and frequency along a constrained trend line governed by the promoter. In summary, transcriptional bursting dominates across the human genome, both burst frequency and burst size vary by chromosomal location, and transcriptional activators alter burst frequency and burst size, depending on the expression level of the locus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

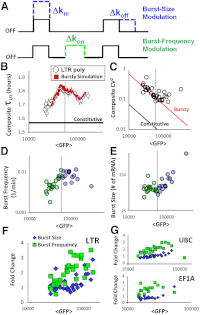
 , fold changes in burst size and frequency are comparable, with an initial increase of frequency in all promoters investigated.
, fold changes in burst size and frequency are comparable, with an initial increase of frequency in all promoters investigated.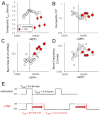
References
-
- Yunger S, Rosenfeld L, Garini Y, Shav-Tal Y. Single-allele analysis of transcription kinetics in living mammalian cells. Nat Methods. 2010;7:631–633. - PubMed
-
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. - PubMed
-
- Pedraza JM, Paulsson J. Effects of molecular memory and bursting on fluctuations in gene expression. Science. 2008;319:339–343. - PubMed
-
- Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440:358–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

