Rituximab-treated patients have a poor response to influenza vaccination
- PMID: 23064976
- PMCID: PMC3565069
- DOI: 10.1007/s10875-012-9813-x
Rituximab-treated patients have a poor response to influenza vaccination
Abstract
The efficacy of influenza vaccination in patients treated with rituximab is a clinically important question. Rheumatology clinics are populated with patients receiving rituximab for a broad array of disorders. Although several studies have explored the efficacy of other vaccines in rituximab-treated populations, results have been conflicting. We wished to define influenza vaccine efficacy in a rituximab-treated cohort. We examined 17 evaluable subjects treated with rituximab for rheumatologic conditions. T cell subsets, B cells subsets, T cell function, and B cell function were evaluated at specific time points along with hemagglutinination inhibition titers after receiving the standard inactivated influenza vaccine. T cell subset counts were significantly different than controls but did not change with rituximab. B cells depleted in all patients but were in various stages of recovery at the time of vaccination. Influenza vaccine responsiveness was poor overall, with only 16 % of subjects having a four-fold increase in titer. Pre-existing titers were retained throughout the study, however. The ability to respond to the influenza vaccine appeared to be related to the degree of B cell recovery at the time of vaccination. This study emphasizes that antibody responses to vaccine are impaired in subjects treated with rituximab and supports the concept that B cell recovery influences influenza vaccine responsiveness.
Figures
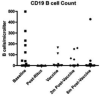


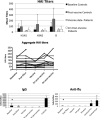
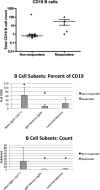
References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi:joc21709 [pii] - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. - PubMed
-
- Neuzil KM, Wright PF, Mitchel EF, Jr., Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137(6):856–864. doi:S0022-3476(00)25105-6 [pii] 10.1067/mpd.2000.110445. - PubMed
-
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr., Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342(4):225–231. doi:MJBA-420401 [pii] - PubMed
-
- Neuzil KM, Reed GW, Mitchel EF, Jr., Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281(10):901–907. doi:joc81147 [pii] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

