Mitochondria as a drug target in ischemic heart disease and cardiomyopathy
- PMID: 23065345
- PMCID: PMC3507431
- DOI: 10.1161/CIRCRESAHA.112.265660
Mitochondria as a drug target in ischemic heart disease and cardiomyopathy
Abstract
Ischemic heart disease is a significant cause of morbidity and mortality in Western society. Although interventions, such as thrombolysis and percutaneous coronary intervention, have proven efficacious in ischemia and reperfusion injury, the underlying pathological process of ischemic heart disease, laboratory studies suggest further protection is possible, and an expansive research effort is aimed at bringing new therapeutic options to the clinic. Mitochondrial dysfunction plays a key role in the pathogenesis of ischemia and reperfusion injury and cardiomyopathy. However, despite promising mitochondria-targeted drugs emerging from the laboratory, very few have successfully completed clinical trials. As such, the mitochondrion is a potential untapped target for new ischemic heart disease and cardiomyopathy therapies. Notably, there are a number of overlapping therapies for both these diseases, and as such novel therapeutic options for one condition may find use in the other. This review summarizes efforts to date in targeting mitochondria for ischemic heart disease and cardiomyopathy therapy and outlines emerging drug targets in this field.
Figures
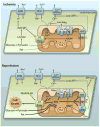

References
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics—2012 Update A Report From the American Heart Association. Circulation. 2012;125:e2–e220. - PMC - PubMed
-
- Stanley WC, Hoppel CL. Mitochondrial Dysfunction in Heart Failure: Potential for Therapeutic Interventions? Cardiovasc Res. 2000;45:805–806. - PubMed
-
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. - PubMed
-
- Opie LH. Metabolism of the heart in health and disease. I. Am Heart J. 1968;76:685–698. - PubMed
-
- Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

