Exacerbated neuronal ceroid lipofuscinosis phenotype in Cln1/5 double-knockout mice
- PMID: 23065637
- PMCID: PMC3597017
- DOI: 10.1242/dmm.010140
Exacerbated neuronal ceroid lipofuscinosis phenotype in Cln1/5 double-knockout mice
Abstract
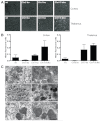
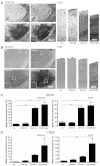
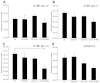
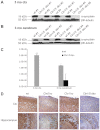
Both CLN1 and CLN5 deficiencies lead to severe neurodegenerative diseases of childhood, known as neuronal ceroid lipofuscinoses (NCLs). The broadly similar phenotypes of NCL mouse models, and the potential for interactions between NCL proteins, raise the possibility of shared or convergent disease mechanisms. To begin addressing these issues, we have developed a new mouse model lacking both Cln1 and Cln5 genes. These double-knockout (Cln1/5 dko) mice were fertile, showing a slight decrease in expected Mendelian breeding ratios, as well as impaired embryoid body formation by induced pluripotent stem cells derived from Cln1/5 dko fibroblasts. Typical disease manifestations of the NCLs, i.e. seizures and motor dysfunction, were detected at the age of 3 months, earlier than in either single knockout mouse. Pathological analyses revealed a similar exacerbation and earlier onset of disease in Cln1/5 dko mice, which exhibited a pronounced accumulation of autofluorescent storage material. Cortical demyelination and more pronounced glial activation in cortical and thalamic regions was followed by cortical neuron loss. Alterations in lipid metabolism in Cln1/5 dko showed a specific increase in plasma phospholipid transfer protein (PLTP) activity. Finally, gene expression profiling of Cln1/5 dko cortex revealed defects in myelination and immune response pathways, with a prominent downregulation of α-synuclein in Cln1/5 dko mouse brains. The simultaneous loss of both Cln1 and Cln5 genes might enhance the typical pathological phenotypes of these mice by disrupting or downregulating shared or convergent pathogenic pathways, which could potentially include interactions of CLN1 and CLN5.
Figures



References
-
- Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W. H., Castillo P. E., Shinsky N., Verdugo J. M., Armanini M., Ryan A., et al. (2000). Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239-252 - PubMed
-
- Ahtiainen L., Kolikova J., Mutka A. L., Luiro K., Gentile M., Ikonen E., Khiroug L., Jalanko A., Kopra O. (2007). Palmitoyl protein thioesterase 1 (Ppt1)-deficient mouse neurons show alterations in cholesterol metabolism and calcium homeostasis prior to synaptic dysfunction. Neurobiol. Dis. 28, 52-64 - PubMed
-
- Bible E., Gupta P., Hofmann S. L., Cooper J. D. (2004). Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 16, 346-359 - PubMed
-
- Camp L. A., Hofmann S. L. (1993). Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J. Biol. Chem. 268, 22566-22574 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

