Enzymatic characterization of AMP phosphorylase and ribose-1,5-bisphosphate isomerase functioning in an archaeal AMP metabolic pathway
- PMID: 23065974
- PMCID: PMC3510611
- DOI: 10.1128/JB.01335-12
Enzymatic characterization of AMP phosphorylase and ribose-1,5-bisphosphate isomerase functioning in an archaeal AMP metabolic pathway
Abstract
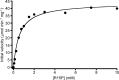
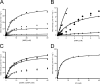
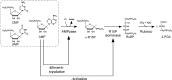
AMP phosphorylase (AMPpase), ribose-1,5-bisphosphate (R15P) isomerase, and type III ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) have been proposed to constitute a novel pathway involved in AMP metabolism in the Archaea. Here we performed a biochemical examination of AMPpase and R15P isomerase from Thermococcus kodakarensis. R15P isomerase was specific for the α-anomer of R15P and did not recognize other sugar compounds. We observed that activity was extremely low with the substrate R15P alone but was dramatically activated in the presence of AMP. Using AMP-activated R15P isomerase, we reevaluated the substrate specificity of AMPpase. AMPpase exhibited phosphorylase activity toward CMP and UMP in addition to AMP. The [S]-v plot (plot of velocity versus substrate concentration) of the enzyme toward AMP was sigmoidal, with an increase in activity observed at concentrations higher than approximately 3 mM. The behavior of the two enzymes toward AMP indicates that the pathway is intrinsically designed to prevent excess degradation of intracellular AMP. We further examined the formation of 3-phosphoglycerate from AMP, CMP, and UMP in T. kodakarensis cell extracts. 3-Phosphoglycerate generation was observed from AMP alone, and from CMP or UMP in the presence of dAMP, which also activates R15P isomerase. 3-Phosphoglycerate was not formed when 2-carboxyarabinitol 1,5-bisphosphate, a Rubisco inhibitor, was added. The results strongly suggest that these enzymes are actually involved in the conversion of nucleoside monophosphates to 3-phosphoglycerate in T. kodakarensis.
Figures


References
-
- Ashida H, et al. 2003. A functional link between RuBisCO-like protein of Bacillus and photosynthetic RuBisCO. Science 302:286–290 - PubMed
-
- Bose R, Yamada EW. 1974. Uridine phosphorylase, molecular properties and mechanism of catalysis. Biochemistry 13:2051–2056 - PubMed
-
- Bumann M, et al. 2004. Crystal structure of yeast Ypr118w, a methylthioribose-1-phosphate isomerase related to regulatory eIF2B subunits. J. Biol. Chem. 279:37087–37094 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

