Evolutionary approaches to prolong progression-free survival in breast cancer
- PMID: 23066036
- PMCID: PMC3525750
- DOI: 10.1158/0008-5472.CAN-12-2235
Evolutionary approaches to prolong progression-free survival in breast cancer
Abstract
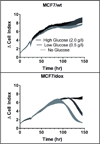
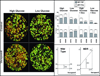
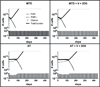
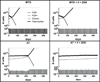
Many cancers adapt to chemotherapeutic agents by upregulating membrane efflux pumps that export drugs from the cytoplasm, but this response comes at an energetic cost. In breast cancer patients, expression of these pumps is low in tumors before therapy but increases after treatment. While the evolution of therapeutic resistance is virtually inevitable, proliferation of resistant clones is not, suggesting strategies of adaptive therapy. Chemoresistant cells must consume excess resources to maintain resistance mechanisms, so adaptive therapy strategies explicitly aim to maintain a stable population of therapy-sensitive cells to suppress growth of resistant phenotypes through intratumoral competition. We used computational models parameterized by in vitro experiments to illustrate the efficacy of such approaches. Here, we show that low doses of verapamil and 2-deoxyglucose, to accentuate the cost of resistance and to decrease energy production, respectively, could suppress the proliferation of drug-resistant clones in vivo. Compared with standard high-dose-density treatment, the novel treatment we developed achieved a 2-fold to 10-fold increase in time to progression in tumor models. Our findings challenge the existing flawed paradigm of maximum dose treatment, a strategy that inevitably produces drug resistance that can be avoided by the adaptive therapy strategies we describe.
Conflict of interest statement
Conflict of interest: The authors have no conflicts of interest to disclose.
Figures

References
-
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. - PubMed
-
- Grogan TM, Spier CM, Salmon SE, Matzner M, Rybski J, Weinstein RS, et al. P-glycoprotein expression in human plasma cell myeloma: correlation with prior chemotherapy. Blood. 1993;81:490–495. - PubMed
-
- Sonneveld P, Schoester M, de Leeuw K. Clinical modulation of multidrug resistance in multiple myeloma: effect of cyclosporine on resistant tumor cells. J Clin Oncol. 1994;12:1584–1591. - PubMed
-
- Troutman MD, Thakker DR. Novel experimental parameters to quantify the modulation of absorptive and secretory transport of compounds by P-glycoprotein in cell culture models of intestinal epithelium. Pharm Res. 2003;20:1210–1224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

