Myeloperoxidase: a front-line defender against phagocytosed microorganisms
- PMID: 23066164
- PMCID: PMC3545676
- DOI: 10.1189/jlb.0712349
Myeloperoxidase: a front-line defender against phagocytosed microorganisms
Abstract
Successful immune defense requires integration of multiple effector systems to match the diverse virulence properties that members of the microbial world might express as they initiate and promote infection. Human neutrophils--the first cellular responders to invading microbes--exert most of their antimicrobial activity in phagosomes, specialized membrane-bound intracellular compartments formed by ingestion of microorganisms. The toxins generated de novo by the phagocyte NADPH oxidase and delivered by fusion of neutrophil granules with nascent phagosomes create conditions that kill and degrade ingested microbes. Antimicrobial activity reflects multiple and complex synergies among the phagosomal contents, and optimal action relies on oxidants generated in the presence of MPO. The absence of life-threatening infectious complications in individuals with MPO deficiency is frequently offered as evidence that the MPO oxidant system is ancillary rather than essential for neutrophil-mediated antimicrobial activity. However, that argument fails to consider observations from humans and KO mice that demonstrate that microbial killing by MPO-deficient cells is less efficient than that of normal neutrophils. We present evidence in support of MPO as a major arm of oxidative killing by neutrophils and propose that the essential contribution of MPO to normal innate host defense is manifest only when exposure to pathogens overwhelms the capacity of other host defense mechanisms.
Figures
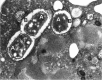


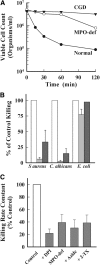


References
-
- Klebanoff S. J. (2005) Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

