A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals
- PMID: 23066504
- PMCID: PMC3463246
- DOI: 10.7554/eLife.00013
A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals
Abstract
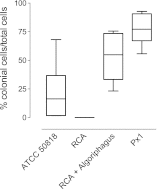
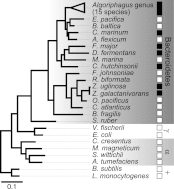
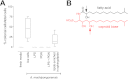
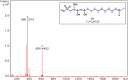
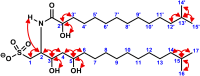
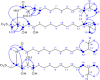
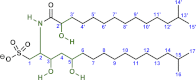
Bacterially-produced small molecules exert profound influences on animal health, morphogenesis, and evolution through poorly understood mechanisms. In one of the closest living relatives of animals, the choanoflagellate Salpingoeca rosetta, we find that rosette colony development is induced by the prey bacterium Algoriphagus machipongonensis and its close relatives in the Bacteroidetes phylum. Here we show that a rosette inducing factor (RIF-1) produced by A. machipongonensis belongs to the small class of sulfonolipids, obscure relatives of the better known sphingolipids that play important roles in signal transmission in plants, animals, and fungi. RIF-1 has extraordinary potency (femtomolar, or 10(-15) M) and S. rosetta can respond to it over a broad dynamic range-nine orders of magnitude. This study provides a prototypical example of bacterial sulfonolipids triggering eukaryotic morphogenesis and suggests molecular mechanisms through which bacteria may have contributed to the evolution of animals.DOI:http://dx.doi.org/10.7554/eLife.00013.001.
Keywords: Algoriphagus; Other; Salpingoeca rosetta; bacterial sulfonolipid; multicellular development.
Conflict of interest statement
JC: Reviewing Editor,
The remaining authors have no competing interests to declare.
Figures





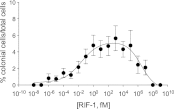


Comment in
- doi: 10.7554/eLife.00242
References
-
- Agosta WC. 1992. Chemical communication. Scientific American Press, New York
-
- Ahmed I, Yokota A, Fujiwara T. 2007. Chimaereicella boritolerans sp nov., a boron-tolerant and alkaliphilic bacterium of the family Flavobacteriaceae isolated from soil. Int J Syst Evol Microbiol 57: 986–992 - PubMed
-
- Anton J, Oren A, Benlloch S, Rodriguez-Valera F, Amann R, Rossello-Mora R. 2002. Salinibacter ruber gen. nov., sp nov., a novel, extremely halophilic member of the bacteria from saltern crystallizer ponds. Int J Syst Evol Microbiol 52: 485–491 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

