Functional asymmetry in kinesin and dynein dimers
- PMID: 23066835
- PMCID: PMC3535566
- DOI: 10.1111/boc.201200044
Functional asymmetry in kinesin and dynein dimers
Abstract
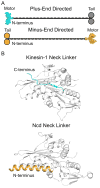
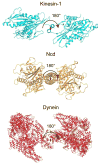
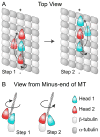
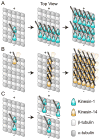
Active transport along the microtubule lattice is a complex process that involves both the Kinesin and Dynein superfamily of motors. Transportation requires sophisticated regulation much of which occurs through the motor's tail domain. However, a significant portion of this regulation also occurs through structural changes that arise in the motor and the microtubule upon binding. The most obvious structural change being the manifestation of asymmetry. To a first approximation in solution, kinesin dimers exhibit twofold symmetry, and microtubules exhibit helical symmetry. The higher symmetries of both the kinesin dimers and microtubule lattice are lost on formation of the kinesin-microtubule complex. Loss of symmetry has functional consequences such as an asymmetric hand-over-hand mechanism in plus-end-directed kinesins, asymmetric microtubule binding in the Kinesin-14 family, spatially biased stepping in dynein and cooperative binding of additional motors to the microtubule. This review focusses on how the consequences of asymmetry affect regulation of motor heads within a dimer, dimers within an ensemble of motors, and suggests how these asymmetries may affect regulation of active transport within the cell.
Copyright © 2013 Soçiété Française des Microscopies and Soçiété de Biologie Cellulaire de France.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

