CHANCE: comprehensive software for quality control and validation of ChIP-seq data
- PMID: 23068444
- PMCID: PMC4053734
- DOI: 10.1186/gb-2012-13-10-r98
CHANCE: comprehensive software for quality control and validation of ChIP-seq data
Abstract
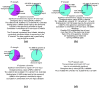
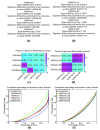
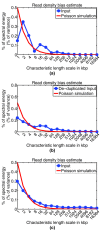
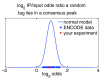
ChIP-seq is a powerful method for obtaining genome-wide maps of protein-DNA interactions and epigenetic modifications. CHANCE (CHip-seq ANalytics and Confidence Estimation) is a standalone package for ChIP-seq quality control and protocol optimization. Our user-friendly graphical software quickly estimates the strength and quality of immunoprecipitations, identifies biases, compares the user's data with ENCODE's large collection of published datasets, performs multi-sample normalization, checks against quantitative PCR-validated control regions, and produces informative graphical reports. CHANCE is available at https://github.com/songlab/chance.
Figures



References
-
- Avardis NGS. http://www.avadis-ngs.com/

