Reduction of intimal hyperplasia in injured rat arteries promoted by catheter balloons coated with polyelectrolyte multilayers that contain plasmid DNA encoding PKCδ
- PMID: 23069712
- PMCID: PMC3483441
- DOI: 10.1016/j.biomaterials.2012.09.010
Reduction of intimal hyperplasia in injured rat arteries promoted by catheter balloons coated with polyelectrolyte multilayers that contain plasmid DNA encoding PKCδ
Abstract
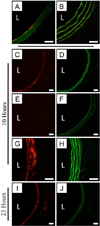
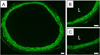
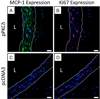
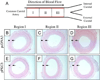
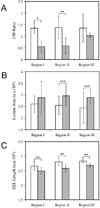
New therapeutic approaches that eliminate or reduce the occurrence of intimal hyperplasia following balloon angioplasty could improve the efficacy of vascular interventions and improve the quality of life of patients suffering from vascular diseases. Here, we report that treatment of arteries using catheter balloons coated with thin polyelectrolyte-based films ('polyelectrolyte multilayers', PEMs) can substantially reduce intimal hyperplasia in an in vivo rat model of vascular injury. We used a layer-by-layer (LbL) process to coat the surfaces of inflatable catheter balloons with PEMs composed of nanolayers of a cationic poly(β-amino ester) (polymer 1) and plasmid DNA (pPKCδ) encoding the δ isoform of protein kinase C (PKCδ), a regulator of apoptosis and other cell processes that has been demonstrated to reduce intimal hyperplasia in injured arterial tissue when administered via perfusion using viral vectors. Insertion of balloons coated with polymer 1/pPKCδ multilayers into injured arteries for 20 min resulted in local transfer of DNA and elevated levels of PKCδ expression in the media of treated tissue three days after delivery. IFC and IHC analysis revealed these levels of expression to promote downstream cellular processes associated with up-regulation of apoptosis. Analysis of arterial tissue 14 days after treatment revealed polymer 1/pPKCδ-coated balloons to reduce the occurrence of intimal hyperplasia by ~60% compared to balloons coated with films containing empty plasmid vectors. Our results demonstrate the potential therapeutic value of this nanotechnology-based approach to local gene delivery in the clinically important context of balloon-mediated vascular interventions. These PEM-based methods could also prove useful for other in vivo applications that require short-term, surface-mediated transfer of plasmid DNA.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- Mueller RL, Sanborn TA. The history of interventional cardiology - cardiac-catheterization, angioplasty, and related interventions. Am Heart J. 1995;129:146–172. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (tasc ii) J Vasc Surg. 2007;45:S5–S67. - PubMed
-
- Liu MW, Roubin GS, King SB. Restenosis after coronary angioplasty - potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–1387. - PubMed
-
- Elezi S, Kastrati A, Hadamitzky M, Dirschinger J, Neumann FJ, Schomig A. Clinical and angiographic follow-up after balloon angioplasty with provisional stenting for coronary in-stent restenosis. Catheter Cardiovasc Interv. 1999;48:151–156. - PubMed
-
- Sharif F, Daly K, Crowley J, O'Brien T. Current status of catheter- and stent-based gene therapy. Cardiovasc Res. 2004;64:208–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

