T cells redirected to EphA2 for the immunotherapy of glioblastoma
- PMID: 23070117
- PMCID: PMC3589173
- DOI: 10.1038/mt.2012.210
T cells redirected to EphA2 for the immunotherapy of glioblastoma
Abstract
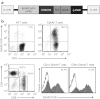
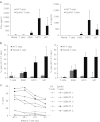
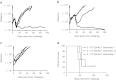
Outcomes for patients with glioblastoma (GBM) remain poor despite aggressive multimodal therapy. Immunotherapy with genetically modified T cells expressing chimeric antigen receptors (CARs) targeting interleukin (IL)-13Rα2, epidermal growth factor receptor variant III (EGFRvIII), or human epidermal growth factor receptor 2 (HER2) has shown promise for the treatment of gliomas in preclinical models and in a clinical study (IL-13Rα2). However, targeting IL-13Rα2 and EGFRvIII is associated with the development of antigen loss variants, and there are safety concerns with targeting HER2. Erythropoietin-producing hepatocellular carcinoma A2 (EphA2) has emerged as an attractive target for the immunotherapy of GBM as it is overexpressed in glioma and promotes its malignant phenotype. To generate EphA2-specific T cells, we constructed an EphA2-specific CAR with a CD28-ζ endodomain. EphA2-specific T cells recognized EphA2-positive glioma cells as judged by interferon-γ (IFN-γ) and IL-2 production and tumor cell killing. In addition, EphA2-specific T cells had potent activity against human glioma-initiating cells preventing neurosphere formation and destroying intact neurospheres in coculture assays. Adoptive transfer of EphA2-specific T cells resulted in the regression of glioma xenografts in severe combined immunodeficiency (SCID) mice and a significant survival advantage in comparison to untreated mice and mice treated with nontransduced T cells. Thus, EphA2-specific T-cell immunotherapy may be a promising approach for the treatment of EphA2-positive GBM.
Figures



References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC.et al. (2009Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial Lancet Oncol 10459–466. - PubMed
-
- Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE.et al. (2011Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma J Clin Oncol 29330–336. - PMC - PubMed
-
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS.et al. (2010Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma J Clin Oncol 284722–4729. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

