Short-term monotherapy with IDX184, a liver-targeted nucleotide polymerase inhibitor, in patients with chronic hepatitis C virus infection
- PMID: 23070151
- PMCID: PMC3497200
- DOI: 10.1128/AAC.01521-12
Short-term monotherapy with IDX184, a liver-targeted nucleotide polymerase inhibitor, in patients with chronic hepatitis C virus infection
Abstract
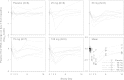
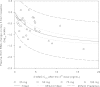
IDX184 is a liver-targeted prodrug of 2'-methylguanosine (2'-MeG) monophosphate. This study investigated the safety, tolerability, antiviral activity, and pharmacokinetics of IDX184 as a single agent in treatment-naïve patients with genotype-1 chronic hepatitis C virus (HCV) infection. Forty-one patients with baseline HCV RNA ≥ 5 log(10) IU/ml, alanine aminotransferase (ALT) ≤ 2.5× the upper limit of normal, and compensated liver disease were dosed. Sequential cohorts of 10 patients, randomized 8:2 (active:placebo), received 25, 50, 75, and 100 mg of IDX184 once daily for 3 days, with a 14-day follow-up. There were no safety-related treatment discontinuations or serious adverse events. The adverse events and laboratory abnormalities observed for IDX184- and placebo-treated patients were similar. At the end of the 3-day treatment period, changes from baseline in HCV RNA levels (means ± standard deviations) were -0.5 ± 0.6, -0.7 ± 0.2, -0.6 ± 0.3, and -0.7 ± 0.5 log(10) for the 25-, 50-, 75-, and 100-mg doses, respectively, while viral load remained unchanged for the pooled placebo patients (-0.05 ± 0.3 log(10)). Patients with genotype-1a and patients with genotype-1b responded similarly. Serum ALT levels decreased, especially at daily doses ≥ 75 mg. During the posttreatment period, plasma viremia and serum aminotransferase levels returned to near pretreatment levels. No resistance mutations associated with IDX184 were detected. Plasma exposure of IDX184 and its nucleoside metabolite 2'-MeG was dose related and low. Changes in plasma viral load correlated with plasma exposure of 2'-MeG. In conclusion, the results from this proof-of-concept study show that small doses of the liver-targeted prodrug IDX184 were able to deliver significant antiviral activity and support further clinical evaluation of the drug candidate.
Figures

References
-
- Cretton-Scott E, et al. 2008. In vitro antiviral activity and pharmacology of IDX184, a novel and potent inhibitor of HCV replication. J. Hepatol. 48(Suppl 2):S220
-
- European Medicines Agency (EMEA) 2009. Guideline on the clinical evaluation of direct acting antiviral agents intended for treatment of chronic hepatitis C. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- FDA 2010. Chronic hepatitis C virus infection: developing direct-acting antiviral agents for treatment. FDA, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati...
-
- Fried MW, et al. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975–982 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

