Modulation of innate immunity by Toxoplasma gondii virulence effectors
- PMID: 23070557
- PMCID: PMC3689224
- DOI: 10.1038/nrmicro2858
Modulation of innate immunity by Toxoplasma gondii virulence effectors
Abstract
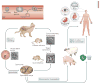
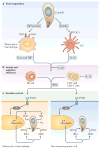
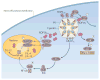
Toxoplasma gondii is a common parasite of animals and humans and can cause serious opportunistic infections. However, the majority of infections are asymptomatic, possibly because the organism has co-evolved with its many vertebrate hosts and has developed multiple strategies to persist asymptomatically for the lifetime of the host. Over the past two decades, infection studies in the mouse, combined with forward-genetics approaches aimed at unravelling the molecular basis of infection, have revealed that T. gondii virulence is mediated, in part, by secretion of effector proteins into the host cell during invasion. Here, we review recent advances that illustrate how these virulence factors disarm innate immunity and promote survival of the parasite.
Figures

References
-
- Dubey JP. Toxoplasmosis of animals and humans. CRC Press; 2010.
-
- Dubey JP, Frenkel JF. Cyst-induced toxoplasmosis in cats. Journal of Protozoology. 1972;19:155–177. - PubMed
-
- Su C, et al. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. - PubMed
-
- Jones JL, Dubey JP. Foodborne Toxoplasmosis. Clin Infect Dis. 2012 cis508 [pii] 10.1093/cid/cis508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

