Evaluation of glutamate concentration transient in the synaptic cleft of the rat calyx of Held
- PMID: 23070699
- PMCID: PMC3630782
- DOI: 10.1113/jphysiol.2012.241398
Evaluation of glutamate concentration transient in the synaptic cleft of the rat calyx of Held
Abstract
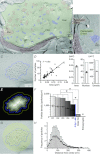
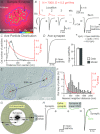
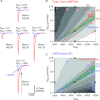
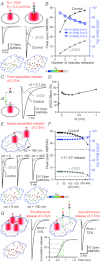
Establishing the spatiotemporal concentration profile of neurotransmitter following synaptic vesicular release is essential for our understanding of inter-neuronal communication. Such profile is a determinant of synaptic strength, short-term plasticity and inter-synaptic crosstalk. Synaptically released glutamate has been suggested to reach a few millimolar in concentration and last for <1 ms. The synaptic cleft is often conceived as a single concentration compartment, whereas a huge gradient likely exists. Modelling studies have attempted to describe this gradient, but two key parameters, the number of glutamate in a vesicle (N(Glu)) and its diffusion coefficient (D(Glu)) in the extracellular space, remained unresolved. To determine this profile, the rat calyx of Held synapse at postnatal day 12-16 was studied where diffusion of glutamate occurs two-dimensionally and where quantification of AMPA receptor distribution on individual postsynaptic specialization on medial nucleus of the trapezoid body principal cells is possible using SDS-digested freeze-fracture replica labelling. To assess the performance of these receptors as glutamate sensors, a kinetic model of the receptors was constructed from outside-out patch recordings. From here, we simulated synaptic responses and compared them with the EPSC recordings. Combinations of N(Glu) and D(Glu) with an optimum of 7000 and 0.3 μm(2) ms(-1) reproduced the data, suggesting slow diffusion. Further simulations showed that a single vesicle does not saturate the synaptic receptors, and that glutamate spillover does not affect the conductance amplitude at this synapse. Using the estimated profile, we also evaluated how the number of multiple vesicle releases at individual active zones affects the amplitude of postsynaptic signals.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

