Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via β-catenin signaling
- PMID: 23070813
- PMCID: PMC3475800
- DOI: 10.1101/gad.196451.112
Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via β-catenin signaling
Abstract
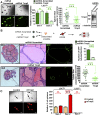
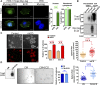
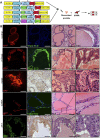
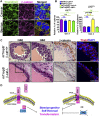
The cell surface protein Trop2 is expressed on immature stem/progenitor-like cells and is overexpressed in many epithelial cancers. However the biological function of Trop2 in tissue maintenance and tumorigenesis remains unclear. In this study, we demonstrate that Trop2 is a regulator of self-renewal, proliferation, and transformation. Trop2 controls these processes through a mechanism of regulated intramembrane proteolysis that leads to cleavage of Trop2, creating two products: the extracellular domain and the intracellular domain. The intracellular domain of Trop2 is released from the membrane and accumulates in the nucleus. Heightened expression of the Trop2 intracellular domain promotes stem/progenitor self-renewal through signaling via β-catenin and is sufficient to initiate precursor lesions to prostate cancer in vivo. Importantly, we demonstrate that loss of β-catenin or Trop2 loss-of-function cleavage mutants abrogates Trop2-driven self-renewal and hyperplasia in the prostate. These findings suggest that heightened expression of Trop2 is selected for in epithelial cancers to enhance the stem-like properties of self-renewal and proliferation. Defining the mechanism of Trop2 function in self-renewal and transformation is essential to identify new therapeutic strategies to block Trop2 activation in cancer.
Figures



References
-
- Alberti S. 2012 Anti-trop-2 monoclonal antibodies and uses thereof in the treatment and diagnosis of tumors. U.S. Patent US 20120052076.
-
- Basu A, Goldenberg DM, Stein R 1995. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int J Cancer 62: 472–479 - PubMed
-
- Bostwick DG, Montironi R 1995. Prostatic intraepithelial neoplasia and the origins of prostatic carcinoma. Pathol Res Pract 191: 828–832 - PubMed
-
- Brown MS, Ye J, Rawson RB, Goldstein JL 2000. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 100: 391–398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
