Heterotrimeric GAIT complex drives transcript-selective translation inhibition in murine macrophages
- PMID: 23071094
- PMCID: PMC3510535
- DOI: 10.1128/MCB.01168-12
Heterotrimeric GAIT complex drives transcript-selective translation inhibition in murine macrophages
Abstract
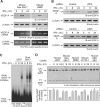
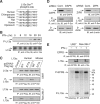
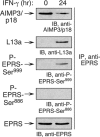
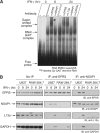
The gamma interferon (IFN-γ)-activated inhibitor of translation (GAIT) complex in human myeloid cells is heterotetrameric, consisting of glutamyl-prolyl-tRNA synthetase (EPRS), NS1-associated protein 1 (NSAP1), ribosomal protein L13a, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The complex binds a structural GAIT element in the 3' untranslated region of VEGF-A and other inflammation-related transcripts and inhibits their translation. EPRS is dually phosphorylated by cyclin-dependent kinase 5 (Cdk5) at Ser(886) and then by a Cdk5-dependent-AGC kinase at Ser(999); L13a is phosphorylated at Ser(77) by death-associated protein kinases DAPK and ZIPK. Because profound differences in inflammatory responses between mice and humans are known, we investigated the GAIT system in mouse macrophages. The murine GAIT complex is heterotrimeric, lacking NSAP1. As in humans, IFN-γ activates the mouse macrophage GAIT system via induced phosphorylation of EPRS and L13a. Murine L13a is phosphorylated at Ser(77) by the DAPK-ZIPK cascade, but EPRS is phosphorylated only at Ser(999). Loss of EPRS Ser(886) phosphorylation prevents NSAP1 incorporation into the GAIT complex. However, the triad of Ser(999)-phosphorylated EPRS, Ser(77)-phosphorylated L13a, and GAPDH forms a functional GAIT complex that inhibits translation of GAIT target mRNAs. Thus, translational control by the heterotrimeric GAIT complex in mice exemplifies the distinctive species-specific responses of myeloid cells to inflammatory stimuli.
Figures



References
-
- Arif A. 2012. Extraneuronal activities and regulatory mechanisms of the atypical cyclin-dependent kinase Cdk5. Biochem. Pharmacol. 84:985–993 - PubMed
-
- Bannai H, et al. 2004. An RNA-interacting protein, SYNCRIP (heterogeneous nuclear ribonuclear protein Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites. J. Biol. Chem. 279:53427–53434 - PubMed
-
- Blanc V, et al. 2001. Identification of GRY-RBP as an apolipoprotein B RNA-binding protein that interacts with both apobec-1 and apobec-1 complementation factor to modulate C to U editing. J. Biol. Chem. 276:10272–10283 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
