HIV-1 Nef impairs heterotrimeric G-protein signaling by targeting Gα(i2) for degradation through ubiquitination
- PMID: 23071112
- PMCID: PMC3510845
- DOI: 10.1074/jbc.M112.361782
HIV-1 Nef impairs heterotrimeric G-protein signaling by targeting Gα(i2) for degradation through ubiquitination
Abstract
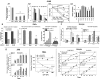
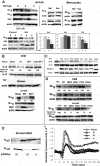
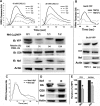
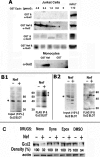
The HIV Nef protein is an important pathogenic factor that modulates cell surface receptor trafficking and impairs cell motility, presumably by interfering at multiple steps with chemotactic receptor signaling. Here, we report that a dominant effect of Nef is to trigger AIP4 E3 ligase-mediated Gα(i2) ubiquitination, which leads to Gα(i2) endolysosomal sequestration and destruction. The loss of the Gα(i2) subunit was demonstrable in many cell types in the context of gene transfection, HIV infection, or Nef protein transduction. Nef directly interacts with Gα(i2) and ternary complexes containing AIP4, Nef, and Gα(i2) form. A substantial reversal of Gα(i2) loss and a partial recovery of impaired chemotaxis occurred following siRNA knockdown of AIP4 or NEDD4 or by inhibiting dynamin. The N-terminal myristoyl group, (62)EEEE(65) motif, and (72)PXXP(75) motif of Nef are critical for this effect to occur. Nef expression does not affect a Gq(i5) chimera where the five C-terminal residues of Gq are replaced with those of Gα(i2). Lysine at position 296 of Gα(i2) was identified as the critical determinant of Nef-induced degradation. By specifically degrading Gα(i2), Nef directly subverts leukocyte migration and homing. Impaired trafficking and homing of HIV Nef-expressing lymphocytes probably contributes to early immune dysfunction following HIV infection.
Figures






Similar articles
-
HIV-1 Nef down-modulates C-C and C-X-C chemokine receptors via ubiquitin and ubiquitin-independent mechanism.PLoS One. 2014 Jan 29;9(1):e86998. doi: 10.1371/journal.pone.0086998. eCollection 2014. PLoS One. 2014. PMID: 24489825 Free PMC article.
-
Identification of G protein alpha subunit-palmitoylating enzyme.Mol Cell Biol. 2009 Jan;29(2):435-47. doi: 10.1128/MCB.01144-08. Epub 2008 Nov 10. Mol Cell Biol. 2009. PMID: 19001095 Free PMC article.
-
Lysine 144, a ubiquitin attachment site in HIV-1 Nef, is required for Nef-mediated CD4 down-regulation.J Immunol. 2008 Jun 15;180(12):7878-86. doi: 10.4049/jimmunol.180.12.7878. J Immunol. 2008. PMID: 18523251
-
Two Functional Variants of AP-1 Complexes Composed of either γ2 or γ1 Subunits Are Independently Required for Major Histocompatibility Complex Class I Downregulation by HIV-1 Nef.J Virol. 2020 Mar 17;94(7):e02039-19. doi: 10.1128/JVI.02039-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915283 Free PMC article.
-
Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: Diverse pathways for G-protein-coupled receptor destruction and beyond.Traffic. 2019 Feb;20(2):101-109. doi: 10.1111/tra.12619. Epub 2018 Nov 18. Traffic. 2019. PMID: 30353650 Free PMC article. Review.
Cited by
-
Proteasomal Degradation Machinery: Favorite Target of HIV-1 Proteins.Front Microbiol. 2018 Nov 21;9:2738. doi: 10.3389/fmicb.2018.02738. eCollection 2018. Front Microbiol. 2018. PMID: 30524389 Free PMC article. Review.
-
An essential role for RGS protein/Gαi2 interactions in B lymphocyte-directed cell migration and trafficking.J Immunol. 2015 Mar 1;194(5):2128-39. doi: 10.4049/jimmunol.1401952. Epub 2015 Jan 23. J Immunol. 2015. PMID: 25617475 Free PMC article.
-
Data and Text Mining Help Identify Key Proteins Involved in the Molecular Mechanisms Shared by SARS-CoV-2 and HIV-1.Molecules. 2020 Jun 26;25(12):2944. doi: 10.3390/molecules25122944. Molecules. 2020. PMID: 32604797 Free PMC article.
-
HIV-1 Balances the Fitness Costs and Benefits of Disrupting the Host Cell Actin Cytoskeleton Early after Mucosal Transmission.Cell Host Microbe. 2019 Jan 9;25(1):73-86.e5. doi: 10.1016/j.chom.2018.12.008. Cell Host Microbe. 2019. PMID: 30629922 Free PMC article.
-
HIV-1 Nef down-modulates C-C and C-X-C chemokine receptors via ubiquitin and ubiquitin-independent mechanism.PLoS One. 2014 Jan 29;9(1):e86998. doi: 10.1371/journal.pone.0086998. eCollection 2014. PLoS One. 2014. PMID: 24489825 Free PMC article.
References
-
- Rossi D., Zlotnik A. (2000) The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 - PubMed
-
- Wurbel M. A. (2000) The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 - PubMed
-
- Kwan J., Killeen N. (2004) CCR7 directs the migration of thymocytes into the thymic medulla. J. Immunol. 172, 3999–4007 - PubMed
-
- Bromley S. K., Thomas S. Y., Luster A. D. (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

