αA-crystallin and αB-crystallin reside in separate subcellular compartments in the developing ocular lens
- PMID: 23071119
- PMCID: PMC3516784
- DOI: 10.1074/jbc.M112.414854
αA-crystallin and αB-crystallin reside in separate subcellular compartments in the developing ocular lens
Abstract
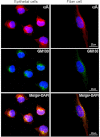
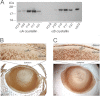
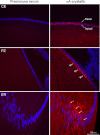
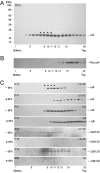
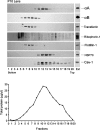
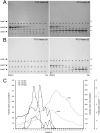
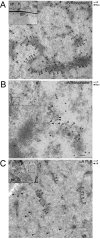
αA-Crystallin (αA) and αB-crystallin (αB), the two prominent members of the small heat shock family of proteins are considered to be two subunits of one multimeric protein, α-crystallin, within the ocular lens. Outside of the ocular lens, however, αA and αB are known to be two independent proteins, with mutually exclusive expression in many tissues. This dichotomous view is buoyed by the high expression of αA and αB in the lens and their co-fractionation from lens extracts as one multimeric entity, α-crystallin. To understand the biological function(s) of each of these two proteins, it is important to investigate the biological basis of this perceived dichotomy; in this report, we address the question whether αA and αB exist as independent proteins in the ocular lens. Discontinuous sucrose density gradient fractionation and immunoconfocal localization reveal that in early developing rat lens αA is a membrane-associated small heat shock protein similar to αB but with remarkable differences. Employing an established protocol, we demonstrate that αB predominantly sediments with rough endoplasmic reticulum, whereas αA fractionates with smooth membranes. These biochemical observations were corroborated with immunogold labeling and transmission electron microscopy. Importantly, in the rat heart also, which does not contain αA, αB fractionates with rough endoplasmic reticulum, suggesting that αA has no influence on the distribution of αB. These data demonstrate presence of αA and αB in two separate subcellular membrane compartments, pointing to their independent existence in the developing ocular lens.
Figures

Similar articles
-
Impact of Subunit Composition on the Uptake of α-Crystallin by Lens and Retina.PLoS One. 2015 Sep 10;10(9):e0137659. doi: 10.1371/journal.pone.0137659. eCollection 2015. PLoS One. 2015. PMID: 26355842 Free PMC article.
-
Elevated expression of alphaA- and alphaB-crystallins in streptozotocin-induced diabetic rat.Arch Biochem Biophys. 2005 Dec 15;444(2):77-83. doi: 10.1016/j.abb.2005.09.021. Epub 2005 Nov 8. Arch Biochem Biophys. 2005. PMID: 16309625
-
Significance of alpha-crystallin heteropolymer with a 3:1 alphaA/alphaB ratio: chaperone-like activity, structure and hydrophobicity.Biochem J. 2008 Sep 15;414(3):453-60. doi: 10.1042/BJ20080544. Biochem J. 2008. PMID: 18479247
-
Regulation of αA- and αB-crystallins via phosphorylation in cellular homeostasis.Cell Mol Life Sci. 2015 Nov;72(21):4127-37. doi: 10.1007/s00018-015-1996-x. Epub 2015 Jul 26. Cell Mol Life Sci. 2015. PMID: 26210153 Free PMC article. Review.
-
Contrast Functions of αA- and αB-Crystallins in Cancer Development.Curr Mol Med. 2017;16(10):914-922. doi: 10.2174/1566524016666161223110508. Curr Mol Med. 2017. PMID: 28017134 Review.
Cited by
-
Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death.Biochim Biophys Acta. 2014 Feb;1843(2):309-15. doi: 10.1016/j.bbamcr.2013.11.010. Epub 2013 Nov 22. Biochim Biophys Acta. 2014. PMID: 24275510 Free PMC article.
-
Lens Biology and Biochemistry.Prog Mol Biol Transl Sci. 2015;134:169-201. doi: 10.1016/bs.pmbts.2015.04.007. Epub 2015 Jun 4. Prog Mol Biol Transl Sci. 2015. PMID: 26310155 Free PMC article. Review.
-
Inhibition of the Expression of the Small Heat Shock Protein αB-Crystallin Inhibits Exosome Secretion in Human Retinal Pigment Epithelial Cells in Culture.J Biol Chem. 2016 Jun 17;291(25):12930-42. doi: 10.1074/jbc.M115.698530. Epub 2016 Apr 27. J Biol Chem. 2016. PMID: 27129211 Free PMC article.
-
Transcriptional regulation of crystallin, redox, and apoptotic genes by C-Phycocyanin in the selenite-induced cataractogenic rat model.Mol Vis. 2015 Jan 14;21:26-39. eCollection 2015. Mol Vis. 2015. PMID: 25593511 Free PMC article.
-
Spatial Analysis of Single Fiber Cells of the Developing Ocular Lens Reveals Regulated Heterogeneity of Gene Expression.iScience. 2018 Dec 21;10:66-79. doi: 10.1016/j.isci.2018.11.024. Epub 2018 Nov 17. iScience. 2018. PMID: 30508719 Free PMC article.
References
-
- Andley U. P. (2007) Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 26, 78–98 - PubMed
-
- Bhat S. P. (2003) Crystallins, genes, and cataract. Prog. Drug. Res. 60, 205–262 - PubMed
-
- Groenen P. J., Merck K. B., de Jong W. W., Bloemendal H. (1994) Structure and modifications of the junior chaperone α-crystallin. From lens transparency to molecular pathology. Eur. J. Biochem. 225, 1–19 - PubMed
-
- Horwitz J. (2000) The function of α-crystallin in vision. Semin. Cell Dev. Biol. 11, 53–60 - PubMed
-
- Bhat S. P., Nagineni C. N. (1989) αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem. Biophys. Res. Commun. 158, 319–325 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

