Vitamin D rescues impaired Mycobacterium tuberculosis-mediated tumor necrosis factor release in macrophages of HIV-seropositive individuals through an enhanced Toll-like receptor signaling pathway in vitro
- PMID: 23071135
- PMCID: PMC3536131
- DOI: 10.1128/IAI.00666-12
Vitamin D rescues impaired Mycobacterium tuberculosis-mediated tumor necrosis factor release in macrophages of HIV-seropositive individuals through an enhanced Toll-like receptor signaling pathway in vitro
Abstract
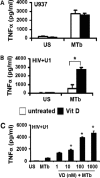
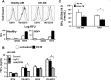
Mycobacterium tuberculosis disease represents an enormous global health problem, with exceptionally high morbidity and mortality in HIV-seropositive (HIV(+)) persons. Alveolar macrophages from HIV(+) persons demonstrate specific and targeted impairment of critical host cell responses, including impaired M. tuberculosis-mediated tumor necrosis factor (TNF) release and macrophage apoptosis. Vitamin D may promote anti-M. tuberculosis responses through upregulation of macrophage NO, NADPH oxidase, cathelicidin, and autophagy mechanisms, but whether vitamin D promotes anti-M. tuberculosis mechanisms in HIV(+) macrophages is not known. In the current study, human macrophages exposed to M. tuberculosis demonstrated robust release of TNF, IκB degradation, and NF-κB nuclear translocation, and these responses were independent of vitamin D pretreatment. In marked contrast, HIV(+) U1 human macrophages exposed to M. tuberculosis demonstrated very low TNF release and no significant IκB degradation or NF-κB nuclear translocation, whereas vitamin D pretreatment restored these critical responses. The vitamin D-mediated restored responses were dependent in part on macrophage CD14 expression. Importantly, similar response patterns were observed with clinically relevant human alveolar macrophages from healthy individuals and asymptomatic HIV(+) persons at high clinical risk of M. tuberculosis infection. Taken together with the observation that local bronchoalveolar lavage fluid (BALF) levels of vitamin D are severely deficient in HIV(+) persons, the data from this study demonstrate that exogenous vitamin D can selectively rescue impaired critical innate immune responses in vitro in alveolar macrophages from HIV(+) persons at risk for M. tuberculosis disease, supporting a potential role for exogenous vitamin D as a therapeutic adjuvant in M. tuberculosis infection in HIV(+) persons.
Figures





References
-
- Anandaiah A, Dheda K, Keane J, Koziel H, Moore DA, Patel NR. 2011. Novel developments in the epidemic of human immunodeficiency virus and tuberculosis coinfection. Am. J. Respir. Crit. Care Med. 183:987–997 doi:10.1164/rccm.201008-1246CI - DOI - PMC - PubMed
-
- WHO 2010. Global tuberculosis control: WHO report 2010. WHO, Geneva, Switzerland
-
- Moreno S, Baraia-Etxaburu J, Bouza E, Parras F, Perez-Tascon M, Miralles P, Vicente T, Alberdi JC, Cosin J, Lopez-Gay D. 1993. Risk for developing tuberculosis among anergic patients infected with HIV. Ann. Intern. Med. 119:194–198 - PubMed
-
- Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. 2005. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191:150–158 - PubMed
-
- Williams BG, Dye C. 2003. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science 301:1535–1537 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

