Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors
- PMID: 23071222
- PMCID: PMC3494837
- DOI: 10.1200/JCO.2012.44.1055
Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors
Abstract
Purpose: Lexatumumab is an agonistic, fully human monoclonal antibody against tumor necrosis factor-related apoptosis-inducing ligand receptor 2 with preclinical evidence of activity in pediatric solid tumors.
Patients and methods: This phase I dose-escalation study examined the safety, tolerability, pharmacokinetics, and immunogenicity of lexatumumab at doses up to, but not exceeding, the adult maximum-tolerated dose (3, 5, 8, and 10 mg/kg), administered once every 2 weeks to patients age≤21 years with recurrent or progressive solid tumors.
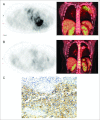
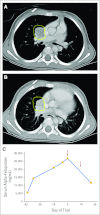
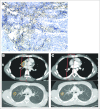
Results: Twenty-four patients received a total of 56 cycles of lexatumumab over all four planned dose levels. One patient had grade 2 pericarditis consistent with radiation recall, and one patient developed grade 3 pneumonia with hypoxia during the second cycle. Five patients experienced stable disease for three to 24 cycles. No patients experienced complete or partial response, but several showed evidence of antitumor activity, including one patient with recurrent progressive osteosarcoma who experienced resolution of clinical symptoms and positron emission tomography activity, ongoing more than 1 year off therapy. One patient with hepatoblastoma showed a dramatic biomarker response.
Conclusion: Pediatric patients tolerate 10 mg/kg of lexatumumab administered once every 14 days, the maximum-tolerated dose identified in adults. The drug seems to mediate some clinical activity in pediatric solid tumors and may work with radiation to enhance antitumor effects.
Trial registration: ClinicalTrials.gov NCT00428272.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
- 4059 doi: 10.1200/JCO.2012.44.9652
References
-
- Cretney E, Takeda K, Yagita H, et al. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. - PubMed
-
- Kontny HU, Hämmerle K, Klein R, et al. Sensitivity of Ewing's sarcoma to TRAIL-induced apoptosis. Cell Death Differ. 2001;8:506–514. - PubMed
-
- Petak I, Douglas L, Tillman DM, et al. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6:4119–4127. - PubMed
-
- Evdokiou A, Bouralexis S, Atkins GJ, et al. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer. 2002;99:491–504. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

