Comparative effectiveness research, genomics-enabled personalized medicine, and rapid learning health care: a common bond
- PMID: 23071236
- PMCID: PMC3504328
- DOI: 10.1200/JCO.2012.42.6114
Comparative effectiveness research, genomics-enabled personalized medicine, and rapid learning health care: a common bond
Abstract
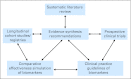
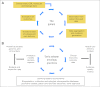
Despite stunning advances in our understanding of the genetics and the molecular basis for cancer, many patients with cancer are not yet receiving therapy tailored specifically to their tumor biology. The translation of these advances into clinical practice has been hindered, in part, by the lack of evidence for biomarkers supporting the personalized medicine approach. Most stakeholders agree that the translation of biomarkers into clinical care requires evidence of clinical utility. The highest level of evidence comes from randomized controlled clinical trials (RCTs). However, in many instances, there may be no RCTs that are feasible for assessing the clinical utility of potentially valuable genomic biomarkers. In the absence of RCTs, evidence generation will require well-designed cohort studies for comparative effectiveness research (CER) that link detailed clinical information to tumor biology and genomic data. CER also uses systematic reviews, evidence-quality appraisal, and health outcomes research to provide a methodologic framework for assessing biologic patient subgroups. Rapid learning health care (RLHC) is a model in which diverse data are made available, ideally in a robust and real-time fashion, potentially facilitating CER and personalized medicine. Nonetheless, to realize the full potential of personalized care using RLHC requires advances in CER and biostatistics methodology and the development of interoperable informatics systems, which has been recognized by the National Cancer Institute's program for CER and personalized medicine. The integration of CER methodology and genomics linked to RLHC should enhance, expedite, and expand the evidence generation required for fully realizing personalized cancer care.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Special series on comparative effectiveness research: challenges to real-world solutions to quality improvement in personalized medicine.J Clin Oncol. 2012 Dec 1;30(34):4188-91. doi: 10.1200/JCO.2012.44.8225. Epub 2012 Oct 15. J Clin Oncol. 2012. PMID: 23071250 No abstract available.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Dancey JE, Bedard PL, Onetto N, et al. The genetic basis for cancer treatment decisions. Cell. 2012;148:409–420. - PubMed
-
- Schilsky RL. Tumor heterogeneity as the foundation of personalized cancer treatment. Semin Oncol. 2011;38:171–172. - PubMed
-
- National Cancer Institute: The National Genome Atlas. http://cancergenome.nih.gov/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

