Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs
- PMID: 23071245
- PMCID: PMC4872304
- DOI: 10.1200/JCO.2012.42.3863
Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs
Erratum in
- J Clin Oncol. 2012 Dec 20;30(36):4590. Ferarri, Maria L [corrected to Ferrari, Maria L]
Abstract
Purpose: Myeloproliferative neoplasm (MPN) symptoms are troublesome to patients, and alleviation of this burden represents a paramount treatment objective in the development of MPN-directed therapies. We aimed to assess the utility of an abbreviated symptom score for the most pertinent and representative MPN symptoms for subsequent serial use in assessing response to therapy.
Patients and methods: The Myeloproliferative Neoplasm Symptom Assessment Form total symptom score (MPN-SAF TSS) was calculated as the mean score for 10 items from two previously validated scoring systems. Questions focus on fatigue, concentration, early satiety, inactivity, night sweats, itching, bone pain, abdominal discomfort, weight loss, and fevers.
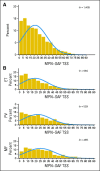
Results: MPN-SAF TSS was calculable for 1,408 of 1,433 patients with MPNs who had a mean score of 21.2 (standard deviation [SD], 16.3). MPN-SAF TSS results significantly differed among MPN disease subtypes (P<.001), with a mean of 18.7 (SD, 15.3), 21.8 (SD, 16.3), and 25.3 (SD, 17.2) for patients with essential thrombocythemia, polycythemia vera, and myelofibrosis, respectively. The MPN-SAF TSS strongly correlated with overall quality of life (QOL; r=0.59; P<.001) and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) functional scales (all P<.001 and absolute r≥0.50 except social functioning r=0.48). No significant trends were present when comparing therapy subgroups. The MPN-SAF TSS had excellent internal consistency (Cronbach's α=.83). Factor analysis identified a single underlying construct, indicating that the MPN-SAF TSS is an appropriate, unified scoring method.
Conclusion: The MPN-SAF TSS is a concise, valid, and accurate assessment of MPN symptom burden with demonstrated clinical utility in the largest prospective MPN symptom study to date. This new prospective scoring method may be used to assess MPN symptom burden in both clinical practice and trial settings.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): An international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. - PubMed
-
- Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): International prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

