Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis
- PMID: 23071254
- PMCID: PMC3478937
- DOI: 10.1084/jem.20120493
Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis
Abstract
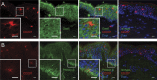
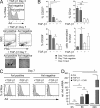
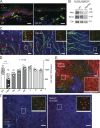
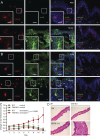
Transforming growth factor-β1 (TGF-β1) is a fundamental regulator of immune cell development and function. In this study, we investigated the effects of TGF-β1 on the differentiation of human Langerhans cells (LCs) and identified Axl as a key TGF-β1 effector. Axl belongs to the TAM (Tyro3, Axl, and Mer) receptor tyrosine kinase family, whose members function as inhibitors of innate inflammatory responses in dendritic cells and are essential to the prevention of lupus-like autoimmunity. We found that Axl expression is induced by TGF-β1 during LC differentiation and that LC precursors acquire Axl early during differentiation. We also describe prominent steady-state expression as well as inflammation-induced activation of Axl in human epidermal keratinocytes and LCs. TGF-β1-induced Axl enhances apoptotic cell (AC) uptake and blocks proinflammatory cytokine production. The antiinflammatory role of Axl in the skin is reflected in a marked impairment of the LC network preceding spontaneous skin inflammation in mutant mice that lack all three TAM receptors. Our findings highlight the importance of constitutive Axl expression to tolerogenic barrier immunity in the epidermis and define a mechanism by which TGF-β1 enables silent homeostatic clearing of ACs to maintain long-term self-tolerance.
Figures





References
-
- Borkowski T.A., Letterio J.J., Farr A.G., Udey M.C. 1996. A role for endogenous transforming growth factor β 1 in Langerhans cell biology: the skin of transforming growth factor β 1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 184:2417–2422 10.1084/jem.184.6.2417 - DOI - PMC - PubMed
-
- Dang H., Geiser A.G., Letterio J.J., Nakabayashi T., Kong L., Fernandes G., Talal N. 1995. SLE-like autoantibodies and Sjögren’s syndrome-like lymphoproliferation in TGF-beta knockout mice. J. Immunol. 155:3205–3212 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

