Evolution of lanthipeptide synthetases
- PMID: 23071302
- PMCID: PMC3494888
- DOI: 10.1073/pnas.1210393109
Evolution of lanthipeptide synthetases
Abstract
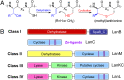
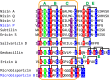
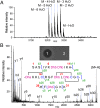
Lanthionine-containing peptides (lanthipeptides) are a family of ribosomally synthesized and posttranslationally modified peptides containing (methyl)lanthionine residues. Here we present a phylogenomic study of the four currently known classes of lanthipeptide synthetases (LanB and LanC for class I, LanM for class II, LanKC for class III, and LanL for class IV). Although they possess very similar cyclase domains, class II-IV synthetases have evolved independently, and LanB and LanC enzymes appear to not always have coevolved. LanM enzymes from various phyla that have three cysteines ligated to a zinc ion (as opposed to the more common Cys-Cys-His ligand set) cluster together. Most importantly, the phylogenomic data suggest that for some scaffolds, the ring topology of the final lanthipeptides may be determined in part by the sequence of the precursor peptides and not just by the biosynthetic enzymes. This notion was supported by studies with two chimeric peptides, suggesting that the nisin and prochlorosin biosynthetic enzymes can produce the correct ring topologies of epilancin 15X and lacticin 481, respectively. These results highlight the potential of lanthipeptide synthetases for bioengineering and combinatorial biosynthesis. Our study also demonstrates unexplored areas of sequence space that may be fruitful for genome mining.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic, machinery, and mechanisms. Chem Rev. 2006;106(8):3468–3496. - PubMed
-
- Strieker M, Tanović A, Marahiel MA. Nonribosomal peptide synthetases: Structures and dynamics. Curr Opin Struct Biol. 2010;20(2):234–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

