Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone
- PMID: 23071308
- PMCID: PMC3497745
- DOI: 10.1073/pnas.1211946109
Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone
Abstract
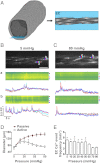
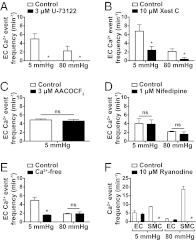
Endothelial cell (EC) Ca(2+)-activated K channels (SK(Ca) and IK(Ca) channels) generate hyperpolarization that passes to the adjacent smooth muscle cells causing vasodilation. IK(Ca) channels focused within EC projections toward the smooth muscle cells are activated by spontaneous Ca(2+) events (Ca(2+) puffs/pulsars). We now show that transient receptor potential, vanilloid 4 channels (TRPV4 channels) also cluster within this microdomain and are selectively activated at low intravascular pressure. In arterioles pressurized to 80 mmHg, ECs generated low-frequency (~2 min(-1)) inositol 1,4,5-trisphosphate receptor-based Ca(2+) events. Decreasing intraluminal pressure below 50 mmHg increased the frequency of EC Ca(2+) events twofold to threefold, an effect blocked with the TRPV4 antagonist RN1734. These discrete events represent both TRPV4-sparklet- and nonsparklet-evoked Ca(2+) increases, which on occasion led to intracellular Ca(2+) waves. The concurrent vasodilation associated with increases in Ca(2+) event frequency was inhibited, and basal myogenic tone was increased, by either RN1734 or TRAM-34 (IK(Ca) channel blocker), but not by apamin (SK(Ca) channel blocker). These data show that intraluminal pressure influences an endothelial microdomain inversely to alter Ca(2+) event frequency; at low pressures the consequence is activation of EC IK(Ca) channels and vasodilation, reducing the myogenic tone that underpins tissue blood-flow autoregulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




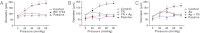
References
-
- Brähler S, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119(17):2323–2332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

