Human cytomegalovirus infection dysregulates the canonical Wnt/β-catenin signaling pathway
- PMID: 23071438
- PMCID: PMC3469659
- DOI: 10.1371/journal.ppat.1002959
Human cytomegalovirus infection dysregulates the canonical Wnt/β-catenin signaling pathway
Abstract
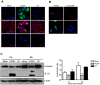
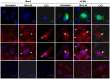
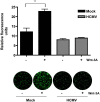
Human Cytomegalovirus (HCMV) is a ubiquitous herpesvirus that currently infects a large percentage of the world population. Although usually asymptomatic in healthy individuals, HCMV infection during pregnancy may cause spontaneous abortions, premature delivery, or permanent neurological disabilities in infants infected in utero. During infection, the virus exerts control over a multitude of host signaling pathways. Wnt/β-catenin signaling, an essential pathway involved in cell cycle control, differentiation, embryonic development, placentation and metastasis, is frequently dysregulated by viruses. How HCMV infection affects this critical pathway is not currently known. In this study, we demonstrate that HCMV dysregulates Wnt/β-catenin signaling in dermal fibroblasts and human placental extravillous trophoblasts. Infection inhibits Wnt-induced transcriptional activity of β-catenin and expression of β-catenin target genes in these cells. HCMV infection leads to β-catenin protein accumulation in a discrete juxtanuclear region. Levels of β-catenin in membrane-associated and cytosolic pools, as well as nuclear β-catenin, are reduced after infection; while transcription of the β-catenin gene is unchanged, suggesting enhanced degradation. Given the critical role of Wnt/β-catenin signaling in cellular processes, these findings represent a novel and important mechanism whereby HCMV disrupts normal cellular function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Steininger C (2007) Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clin Microbiol Infect 13: 953–963. - PubMed
-
- Cannon MJ (2009) Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol 46 (Suppl 4) S6–10. - PubMed
-
- Trincado DE, Rawlinson WD (2001) Congenital and perinatal infections with cytomegalovirus. J Paediatr Child Health 37: 187–192. - PubMed
-
- Demmler GJ (1996) Congenital cytomegalovirus infection and disease. Adv Pediatr Infect Dis 11: 135–162. - PubMed
-
- Nigro G, Adler SP (2011) Cytomegalovirus infections during pregnancy. Curr Opin Obstet Gynecol 23: 123–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

