Phosphatase-dead myotubularin ameliorates X-linked centronuclear myopathy phenotypes in mice
- PMID: 23071445
- PMCID: PMC3469422
- DOI: 10.1371/journal.pgen.1002965
Phosphatase-dead myotubularin ameliorates X-linked centronuclear myopathy phenotypes in mice
Abstract
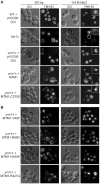
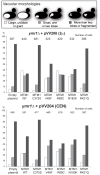
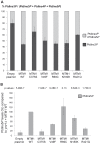
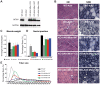
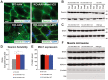
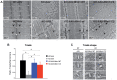
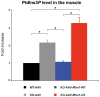
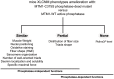
Myotubularin MTM1 is a phosphoinositide (PPIn) 3-phosphatase mutated in X-linked centronuclear myopathy (XLCNM; myotubular myopathy). We investigated the involvement of MTM1 enzymatic activity on XLCNM phenotypes. Exogenous expression of human MTM1 in yeast resulted in vacuolar enlargement, as a consequence of its phosphatase activity. Expression of mutants from patients with different clinical progression and determination of PtdIns3P and PtdIns5P cellular levels confirmed the link between vacuolar morphology and MTM1 phosphatase activity, and showed that some disease mutants retain phosphatase activity. Viral gene transfer of phosphatase-dead myotubularin mutants (MTM1(C375S) and MTM1(S376N)) significantly improved most histological signs of XLCNM displayed by a Mtm1-null mouse, at similar levels as wild-type MTM1. Moreover, the MTM1(C375S) mutant improved muscle performance and restored the localization of nuclei, triad alignment, and the desmin intermediate filament network, while it did not normalize PtdIns3P levels, supporting phosphatase-independent roles of MTM1 in maintaining normal muscle performance and organelle positioning in skeletal muscle. Among the different XLCNM signs investigated, we identified only triad shape and fiber size distribution as being partially dependent on MTM1 phosphatase activity. In conclusion, this work uncovers MTM1 roles in the structural organization of muscle fibers that are independent of its enzymatic activity. This underlines that removal of enzymes should be used with care to conclude on the physiological importance of their activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, et al. (1996) A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13: 175–182. - PubMed
-
- Biancalana V, Caron O, Gallati S, Baas F, Kress W, et al. (2003) Characterisation of mutations in 77 patients with X-linked myotubular myopathy, including a family with a very mild phenotype. Hum Genet 112: 135–142. - PubMed
-
- Laporte J, Biancalana V, Tanner SM, Kress W, Schneider V, et al. (2000) MTM1 mutations in X-linked myotubular myopathy. Hum Mutat 15: 393–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

