The Paramecium germline genome provides a niche for intragenic parasitic DNA: evolutionary dynamics of internal eliminated sequences
- PMID: 23071448
- PMCID: PMC3464196
- DOI: 10.1371/journal.pgen.1002984
The Paramecium germline genome provides a niche for intragenic parasitic DNA: evolutionary dynamics of internal eliminated sequences
Abstract
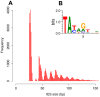
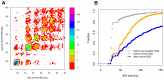
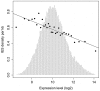
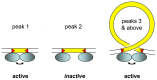
Insertions of parasitic DNA within coding sequences are usually deleterious and are generally counter-selected during evolution. Thanks to nuclear dimorphism, ciliates provide unique models to study the fate of such insertions. Their germline genome undergoes extensive rearrangements during development of a new somatic macronucleus from the germline micronucleus following sexual events. In Paramecium, these rearrangements include precise excision of unique-copy Internal Eliminated Sequences (IES) from the somatic DNA, requiring the activity of a domesticated piggyBac transposase, PiggyMac. We have sequenced Paramecium tetraurelia germline DNA, establishing a genome-wide catalogue of -45,000 IESs, in order to gain insight into their evolutionary origin and excision mechanism. We obtained direct evidence that PiggyMac is required for excision of all IESs. Homology with known P. tetraurelia Tc1/mariner transposons, described here, indicates that at least a fraction of IESs derive from these elements. Most IES insertions occurred before a recent whole-genome duplication that preceded diversification of the P. aurelia species complex, but IES invasion of the Paramecium genome appears to be an ongoing process. Once inserted, IESs decay rapidly by accumulation of deletions and point substitutions. Over 90% of the IESs are shorter than 150 bp and present a remarkable size distribution with a -10 bp periodicity, corresponding to the helical repeat of double-stranded DNA and suggesting DNA loop formation during assembly of a transpososome-like excision complex. IESs are equally frequent within and between coding sequences; however, excision is not 100% efficient and there is selective pressure against IES insertions, in particular within highly expressed genes. We discuss the possibility that ancient domestication of a piggyBac transposase favored subsequent propagation of transposons throughout the germline by allowing insertions in coding sequences, a fraction of the genome in which parasitic DNA is not usually tolerated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Aury J-M, Jaillon O, Duret L, Noel B, Jubin C, et al. (2006) Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 444: 171–178 doi:nature05230 - DOI - PubMed
-
- Chalker DL, Yao M-C (2011) DNA elimination in ciliates: transposon domestication and genome surveillance. Annu Rev Genet 45: 227–246 doi:10.1146/annurev-genet-110410-132432. - PubMed
-
- Coyne RS, Lhuillier-Akakpo M, Duharcourt S (2012) RNA-guided DNA rearrangements in ciliates: is the best genome defense a good offense? Biol Cell Accepted manuscript online doi:10.1111/boc.201100057. - PubMed
-
- Bétermier M (2004) Large-scale genome remodelling by the developmentally programmed elimination of germ line sequences in the ciliate Paramecium. Res Microbiol 155: 399–408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

